Immune checkpoint inhibitor-associated Vogt-Koyanagi-Harada-like syndrome: A descriptive systematic review
- PMID: 40354015
- PMCID: PMC12069190
- DOI: 10.1186/s12348-025-00484-8
Immune checkpoint inhibitor-associated Vogt-Koyanagi-Harada-like syndrome: A descriptive systematic review
Abstract
Topic: Vogt-Koyanagi-Harada (VKH)-like uveitis is uniquely reported with immune checkpoint inhibitors (ICI) and BRAF/MEK inhibitors. This article aims to provide a comprehensive portrait of the comorbidities, ocular presentations, treatments, and visual outcomes of patients with VKH-like uveitis following ICI therapy.
Clinical relevance: ICIs are increasingly used in cancer therapy, but poorly understood ocular immune-related adverse events (irAEs) can lead to suspension of treatment and be vision-threatening.
Methods: We conducted a systematic review (PROSPERO #CRD42024558269) according to PRISMA guidelines. MEDLINE, Embase, CENTRAL, and Web of Science were searched for English articles published up to June 28, 2024. All study designs reporting on incident VKH-like uveitis following ICI were included. Risk of Bias was assessed using a tool modified from Murad et al. (2018).
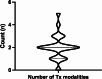
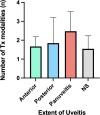
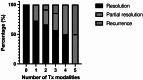
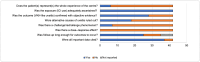
Results: Of 865 articles, we included 42 articles (4 observational studies, 28 case reports, 6 case series, 3 letters, and 1 editorial) from 12 countries, comprising 52 patients. The mean age was 60.0 ± 11.9 years, and 32 (61.5%) were females. Thirty-six (69.2%) had melanoma, and most were undergoing treatment with a PD-1 inhibitor alone (n = 33, 63.5%) or in combination with a CTLA-4 inhibitor (n = 10, 19.2%). The mean duration of ICI treatment before VKH-like uveitis symptoms was 22.2 ± 29.6 weeks, and the mean duration of ocular symptoms was 16.7 ± 18.6 weeks, with wide variation. Overall, 43 patients (73.1%) had imaging or exams suggesting bilateral involvement and 21 cases (40.4%) suggesting panuveitis. Only 31 cases (59.6%) met the acute initial-onset uveitis criteria, and 15 (28.8%) met the chronic phase criteria. Most (n = 47, 90.4%) required systemic or intravitreal steroids, termination of ICI (n = 31, 59.6%), and experienced full resolution or remission of visual symptoms (n = 43, 82.7%). Most articles (n = 40, 95.2%) were judged to be at medium risk of bias.
Conclusion: This descriptive systematic review consisted mostly of case reports, but it confirmed that a high proportion of VKH-like uveitis occur with PD-1 inhibitors and melanoma patients. VKH-like uveitis can lead to suspension of treatment. Further collaboration between oncologists and ophthalmologists is needed in the continuum of cancer care.
Keywords: Drug-induced; Immune checkpoint inhibitor; Immune related adverse event; Panuveitis; Vogt-Koyanagi-Harada-like uveitis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


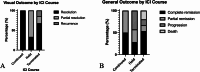
Similar articles
-
Vitiligo as a First Sign of Vogt-Koyanagi-Harada Disease.Acta Dermatovenerol Croat. 2023 Dec;31(4):229-231. Acta Dermatovenerol Croat. 2023. PMID: 38651852
-
HLA-DRB1*04:05 is involved in the development of Vogt-Koyanagi-Harada disease-like immune-related adverse events in patients receiving immune checkpoint inhibitors.Sci Rep. 2023 Aug 21;13(1):13580. doi: 10.1038/s41598-023-40565-z. Sci Rep. 2023. PMID: 37604934 Free PMC article.
-
A Case of Vogt-Koyanagi-Harada Disease-Like Uveitis Induced by Nivolumab and Ipilimumab Combination Therapy.Case Rep Ophthalmol. 2021 Dec 2;12(3):952-960. doi: 10.1159/000520416. eCollection 2021 Sep-Dec. Case Rep Ophthalmol. 2021. PMID: 35082654 Free PMC article.
-
Diagnosing and Managing Uveitis Associated with Immune Checkpoint Inhibitors: A Review.Diagnostics (Basel). 2024 Feb 4;14(3):336. doi: 10.3390/diagnostics14030336. Diagnostics (Basel). 2024. PMID: 38337852 Free PMC article. Review.
-
Vogt-koyanagi-harada syndrome.Curr Eye Res. 2008 Jul;33(7):517-23. doi: 10.1080/02713680802233968. Curr Eye Res. 2008. PMID: 18600484 Review.
References
-
- Alba-Linero C, Alba E (2021) Ocular side effects of checkpoint inhibitors. Surv Ophthalmol 66:951–959. 10.1016/j.survophthal.2021.01.001 - PubMed
-
- Anquetil C, Salem J-E, Lebrun-Vignes B, Touhami S, Desbois A-C, Maalouf G, Domont F, Allenbach Y, Cacoub P, Bodaghi B et al (2020) Evolving spectrum of drug-induced uveitis at the era of immune checkpoint inhibitors results from the who’s pharmacovigilance database. J Autoimmun 111:102454. 10.1016/j.jaut.2020.102454 - PubMed
-
- Herbort CP, Tugal-Tutkun I, Abu-El-Asrar A, Gupta A, Takeuchi M, Fardeau C, Hedayatfar A, Urzua C, Papasavvas I (2022) Precise, simplified diagnostic criteria and optimised management of initial-onset Vogt–Koyanagi–Harada disease: an updated review. Eye (Lond) 36:29–43. 10.1038/s41433-021-01573-3 - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

