Antihypertensive Medication Timing and Cardiovascular Events and Death: The BedMed Randomized Clinical Trial
- PMID: 40354045
- PMCID: PMC12070279
- DOI: 10.1001/jama.2025.4390
Antihypertensive Medication Timing and Cardiovascular Events and Death: The BedMed Randomized Clinical Trial
Abstract
Importance: Whether administration of blood pressure medications at bedtime instead of in the morning reduces cardiovascular risk is unknown, as findings from large clinical trials have not been consistent. There is also concern that bedtime antihypertensive use could induce glaucoma-related visual loss or other hypotensive/ischemic adverse effects.
Objective: To determine the effect of bedtime vs morning administration of antihypertensive medications on major cardiovascular events and death.
Design, setting, and participants: Multicenter, open-label, pragmatic randomized clinical trial with blinded end-point assessment and recruitment via 436 primary care clinicians across 5 Canadian provinces inviting their community-dwelling adult patients with hypertension taking at least 1 once-daily antihypertensive medication. Participants were recruited from March 31, 2017, to May 26, 2022, with final follow-up on December 22, 2023.
Interventions: Participants were randomized in a 1:1 ratio to using all once-daily antihypertensive medications either at bedtime (intervention group; n = 1677) or in the morning (control group; n = 1680).
Main outcomes and measures: The primary outcome was time to first occurrence of all-cause death or hospitalization/emergency department (ED) visit for stroke, acute coronary syndrome, or heart failure. All-cause unplanned hospitalizations/ED visits, and visual, cognitive, and fall- and/or fracture-related safety outcomes were also assessed.
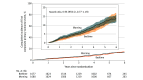
Results: A total of 3357 adults (56.4% female; median age, 67 years; 53.7% taking monotherapy) were randomized and followed up for a median of 4.6 years in each treatment group. The composite primary outcome event occurred at a rate of 2.3 per 100 patient-years in the bedtime group and 2.4 per 100 patient-years in the morning group (adjusted hazard ratio, 0.96; 95% CI, 0.77-1.19; P = .70). Individual components of the primary outcome, all-cause hospitalizations/ED visits, and safety outcomes did not differ between groups. In particular, there was no difference in falls or fractures, new glaucoma diagnoses, or 18-month cognitive decline.
Conclusions and relevance: Among adults with hypertension in primary care, bedtime administration of antihypertensive medications was safe but did not reduce cardiovascular risk. Antihypertensive medication administration time did not affect the risks and benefits of blood pressure-lowering medication and instead should be guided by patient preferences.
Trial registration: ClinicalTrials.gov Identifier: NCT02990663.
Conflict of interest statement
Figures

Comment on
-
Morning or Nighttime Medication Dosing-Does It Matter in the Treatment of Hypertension?JAMA. 2025 Jun 17;333(23):2056-2057. doi: 10.1001/jama.2025.7286. JAMA. 2025. PMID: 40354163 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

