Quantitative Muscle Magnetic Resonance Outcomes in Patients With Duchenne Muscular Dystrophy: An Exploratory Analysis From the EMBARK Randomized Clinical Trial
- PMID: 40354061
- PMCID: PMC12070283
- DOI: 10.1001/jamaneurol.2025.0992
Quantitative Muscle Magnetic Resonance Outcomes in Patients With Duchenne Muscular Dystrophy: An Exploratory Analysis From the EMBARK Randomized Clinical Trial
Abstract
Importance: Delandistrogene moxeparvovec is a recombinant adeno-associated virus rhesus isolate serotype 74 vector-based gene transfer therapy for the treatment of Duchenne muscular dystrophy (DMD) in patients with a confirmed pathogenic variant of the DMD gene. In a subset of patients in the EMBARK (A Gene Transfer Therapy Study to Evaluate the Safety and Efficacy of Delandistrogene Moxeparvovec [SRP-9001] in Participants With DMD) randomized clinical trial, changes in muscle health and pathology were assessed to evaluate the therapeutic impact of the treatment on disease progression.
Objective: To determine the effect of delandistrogene moxeparvovec on muscle quantitative magnetic resonance (QMR) measures of disease progression in patients in the EMBARK trial.
Design, setting, and participants: This was a phase 3, double-blind, placebo-controlled (October 2021-September 2023; week 52 cutoff date: September 13, 2023), multicenter randomized clinical trial that included 131 patients. Patients were randomized, and 125 were treated with either delandistrogene moxeparvovec (n = 63) or placebo (n = 62). The current study focused on a subset of patients who underwent muscle QMR imaging.
Intervention: Single-administration intravenous delandistrogene moxeparvovec (1.33 × 1014 vector genome/kg) or placebo.
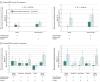
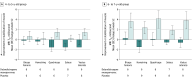
Main outcomes and measures: Change from baseline to week 52 in muscle MR was a prespecified exploratory end point. Proton MR spectroscopy (MRS) and 8-point Dixon MR imaging (MRI) measured muscle fat fraction (FF); multislice spin echo MRI measured transverse relaxation time (T2). MRS FF was measured in the soleus and vastus lateralis. MRI FF and T2 were measured in 5 leg muscle locations important for ambulation. A post hoc global statistical test combining all muscles and modalities assessed overall treatment effect.
Results: In this exploratory EMBARK analysis, 39 male participants (delandistrogene moxeparvovec, n = 19; placebo, n = 20; mean [SD] age, 6.10 [1.04] years; mean [SD] baseline North Star Ambulatory Assessment total score, 22.99 [3.71] points) underwent muscle MRI. Treated patients showed less disease progression vs placebo on MR measures. Across muscles and modalities, magnitudes of FF change favored delandistrogene moxeparvovec; between-group differences in least-squares mean change ranged from -1.01 (95% CI, -2.79 to 0.77; soleus) to -0.71 (95% CI, -3.21 to 1.80; vastus lateralis) for MRS FF and -3.09 (95% CI, -7.62 to 1.45; vastus lateralis) to -0.44 (95% CI, -4.01 to 3.12; hamstrings) for MRI FF. T2 reductions (improvements; 4 of 5 muscles) were observed in treated patients vs increases (worsening; all muscles) in placebo patients; within-group differences in least-squares mean change ranged from -1.06 (95% CI, -2.10 to -0.02; soleus) to 0.17 (95% CI, -1.76 to 2.10; biceps femoris) in the delandistrogene moxeparvovec group and from 1.12 (95% CI, 0.08-2.16; soleus) to 2.94 (95% CI, 0.84-5.03; quadriceps) in the placebo group. The global statistical test supported treatment benefit (P = .03).
Conclusions and relevance: Results reveal that QMR outcomes consistently favored delandistrogene moxeparvovec across muscle groups, with treatment leading to decreased fat accumulation and improved T2 vs placebo over 52 weeks. Consistent with treatment effects on functional outcomes observed in the EMBARK trial, these results suggest stabilization or less progression of muscle pathology with delandistrogene moxeparvovec-adding to the totality of evidence supporting disease stabilization or slowing of disease progression with delandistrogene moxeparvovec.
Trial registration: ClinicalTrials.gov Identifier: NCT05096221.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

