Lecanemab Treatment in a Specialty Memory Clinic
- PMID: 40354064
- PMCID: PMC12070285
- DOI: 10.1001/jamaneurol.2025.1232
Lecanemab Treatment in a Specialty Memory Clinic
Erratum in
-
Errors in Abstract, Discussion, Table 1, Table 2, and Supplement 1.JAMA Neurol. 2025 Jul 1;82(7):754. doi: 10.1001/jamaneurol.2025.2446. JAMA Neurol. 2025. PMID: 40658409 Free PMC article. No abstract available.
Abstract
Importance: Two monoclonal antibodies targeting amyloid plaques, lecanemab and donanemab, have received traditional US Food and Drug Administration (FDA) approval for the treatment of early symptomatic Alzheimer disease (AD). The most significant adverse events associated with these therapies are infusion-related reactions and amyloid-related imaging abnormalities (ARIA) with edema/effusion (ARIA-E) and/or hemorrhage/hemosiderin deposition (ARIA-H). The feasibility and safety of providing these treatments in clinical practice is unclear.
Objective: To examine the feasibility and safety of treating patients in specialty memory clinics with lecanemab.
Design, setting, and participants: This retrospective analysis of consecutive patients in whom lecanemab was initiated between August 1, 2023, and October 1, 2024, at Washington University Memory Diagnostic Center, an outpatient specialty memory clinic. Lecanemab was initiated in 234 patients with early symptomatic AD. Eligibility was based on the FDA label and appropriate use recommendations with occasional exceptions.
Exposure: Patients were treated with lecanemab, 10 mg/kg, intravenously every 2 weeks.
Main outcomes and measures: Infusion-related reactions, ARIA, and withdrawal from treatment were assessed.
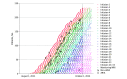
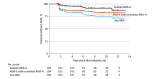
Results: The 234 patients treated with lecanemab had a mean age of 74.4 (SD, 6.7) years, 117 were female (50%), and 117 were male (50%). Infusion-related reactions occurred in 87 patients (37%) and were typically mild. Of the 194 patients at risk for ARIA during the study period, 44 had at least 1 microhemorrhage and/or superficial siderosis before initiation of lecanemab (23%). Over an average treatment period of 6.5 months, 42 total patients (22%) developed ARIA; 29 developed ARIA-E with or without ARIA-H (15%) and 13 developed isolated ARIA-H (6.7%). Eleven patients (5.7%) developed symptomatic ARIA, 2 of those patients (1.0%) with clinically severe symptoms. No patients developed a macrohemorrhage or died. Patients with mild dementia had a 27% rate of symptomatic ARIA; those with mild cognitive impairment or very mild dementia had a 1.8% rate. Overall, 23 of 234 patients (9.8%) withdrew from treatment for various reasons, 10 for ARIA (4.3%).
Conclusions and relevance: A single-specialty memory clinic initiated lecanemab treatment in 234 patients over 14 months. The frequency of significant adverse events, including ARIA, was manageable. These results may inform discussions about the risks of anti-amyloid treatments.
Conflict of interest statement
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

