First-in-Human Study to Evaluate the Safety and Efficacy of Anti-GDF15 Antibody AZD8853 in Patients with Advanced/Metastatic Solid Tumors
- PMID: 40354065
- PMCID: PMC12127903
- DOI: 10.1158/2767-9764.CRC-24-0565
First-in-Human Study to Evaluate the Safety and Efficacy of Anti-GDF15 Antibody AZD8853 in Patients with Advanced/Metastatic Solid Tumors
Abstract
Purpose: Growth and differentiation factor 15 (GDF15) is overexpressed in multiple solid tumors and is thought to exert immunosuppressive effects in the tumor microenvironment. AZD8853 is an anti-GDF15 mAb.
Patients and methods: This first-in-human, phase I/IIa, open-label study (NCT05397171) assessed AZD8853 monotherapy in previously treated patients with advanced/metastatic microsatellite-stable colorectal cancer and urothelial carcinoma. The primary objective was safety including dose-limiting toxicities. Secondary objectives included efficacy, pharmacokinetics, and pharmacodynamics (PD), including free serum GDF15. Exploratory objectives included biomarkers of clinical activity and effects on cancer cachexia.
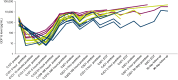
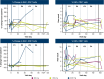
Results: During dose escalation, 16 patients received AZD8853 300 mg (n = 3), 1,000 mg (n = 6), or 3,000 mg (n = 7) intravenously every 3 weeks; 15 patients had microsatellite-stable colorectal cancer; and one patient had urothelial carcinoma. By June 6, 2023, all patients had discontinued treatment. Thirteen (81.3%) patients had treatment-emergent adverse events (TEAE); most commonly diarrhea (31.3%), abdominal pain (31.3%), and decreased appetite (25%). Eight (50.0%) patients had grade ≥3 TEAEs, and six (37.5%) had serious TEAEs, none treatment related. There were no dose-limiting toxicities. The best response per RECIST v1.1 was stable disease in five (31.3%) patients and disease progression in 11 (68.8%) patients. AZD8853 showed linear pharmacokinetics with a half-life of 5 to 10 days, supporting every 3 weeks dosing. AZD8853 suppression of GDF15 was transient. There was no evidence of ctDNA clearance or dose-dependent changes in peripheral T cells. Changes in body weight showed no apparent trends.
Conclusions: AZD8853 was well tolerated; however, no objective responses or PD effects were seen, and GDF15 suppression was not sustained. The study was terminated after dose escalation.
Significance: GDF15 is upregulated in the tumor microenvironment and suppresses antitumor immune responses. In this first-in-human trial, the anti-GDF15 antibody AZD8853 was well tolerated in previously treated patients with advanced/metastatic solid tumors, but no objective responses or PD effects were seen and GDF15 suppression was not sustained.
©2025 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
B.A. Carneiro reports other from AstraZeneca during the conduct of the study, as well as other support from AbbVie Inc., Actuate Therapeutics, Agenus, Astellas Pharma, Bayer, Daiichi Sankyo, Dragonfly Therapeutics, Pfizer, Pyxis Oncology, Regeneron, Repare Therapeutics, and MiNK Therapeutics and personal fees from Eisai, ADC Therapeutics, and Seattle Genetics outside the submitted work. O.B. Gbolahan reports other from AstraZeneca during the conduct of the study, as well as other support from AstraZeneca, Pfizer, Lexicon, Astellas Pharma, Exelixis, QED Therapeutics, Incyte, Eisai, Genentech, Relay Therapeutics, and Novartis outside the submitted work. A.A. Abdul Razak reports grants from AstraZeneca during the conduct of the study, as well as personal fees from Alexion outside the submitted work. J.F. Hilton reports personal fees from Bristol Myers Squibb, Gilead Sciences, Pfizer, and Novartis and grants from GlaxoSmithKline outside the submitted work. A.W. Lambert reports personal fees from AstraZeneca during the conduct of the study, as well as personal fees from AstraZeneca outside the submitted work. J. Hood reports employment with AstraZeneca (this was an AstraZeneca clinical trial done during his employment with AstraZeneca, and AstraZeneca owns patents for AZD8853). M. Pluta reports personal fees from AstraZeneca UK during the conduct of the study, as well as personal fees from AstraZeneca UK outside the submitted work. E. Sanai reports personal fees and other support from AstraZeneca outside the submitted work. R. Kumar reports other support from AstraZeneca outside the submitted work. D.I. Jodrell reports other support from AstraZeneca during the conduct of the study, as well as grants and nonfinancial support from AstraZeneca outside the submitted work. P.M. LoRusso reports other support from Takeda, SOTIO, Agenus, Pfizer, GlaxoSmithKline, Kyowa Kirin Pharmaceutical Development, Kineta Inc., Molecular Templates, I-MAB, MEKanistic Therapeutics, Actuate Therapeutics, Atreca Inc., Amgen CodeBreaK 202, Cullinan, Dren Bio, Quanta Therapeutics, Schrodinger, Boehringer Ingelheim, Prelude, Wells Therapeutics, Zai Lab, and EMAD Serono outside the submitted work. No disclosures were reported by the other authors.
Figures


References
-
- Hurt E, Thomas S, Mulgrew K, Blackmore S, Moynihan J, Cusdin F, et al. . Abstract 1828: AZD8853: a novel antibody targeting GDF15 for immunotherapy refractory tumors. Cancer Res 2021;81(Suppl 13):1828.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

