Living myocardial slices: walking the path towards standardization
- PMID: 40354127
- PMCID: PMC12236068
- DOI: 10.1093/cvr/cvaf079
Living myocardial slices: walking the path towards standardization
Abstract
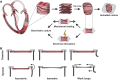
Cardiovascular disease remains a persistent global health burden, underscoring the necessity for effective therapeutic strategies. Despite significant advances, the ability to mechanistically study human disease and predict clinical outcomes remains limited, especially in complex diseases such as heart failure. This limitation is evident through the continuous high attrition rates in drug development pipelines. To address these challenges and contribute to improved preclinical studies, there is a need for platforms that more accurately recapitulate the human heart. This need increased the interest in living myocardial slices (LMS) - thin sections of the heart of approximately 100-400 μm. LMS retain the native multicellular architecture of the heart and enable extended ex vivo culture. However, as their utilization grows, so does variability in preparation methodologies and readouts. This review provides an overview of differences in sample selection, interspecies variations, intra-cardiac differences, and potential confounding factors. Additionally, we examine culture methods, addressing electrical and mechanical stimulation differences, and medium compositions. Our review concludes by highlighting the current limitations of LMS research and offers guidelines for standardization and future applications. The ultimate aim of this review is to serve as a resource for researchers working with LMS and for those entering this field. By presenting the landscape of methodological considerations, we aim to facilitate informed decision-making in study design and execution. We advocate for accurate reporting of methodologies to promote reproducibility and comparability across studies, advancing LMS research and strengthening its role as a valuable addition to the current drug development toolbox and basic cardiovascular research.
Keywords: Disease modeling; Living myocardial slices; Mechanical and electrical stimulation; Metabolism; Translational research.
© The Author(s) 2025. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: A.D. is a shareholder of InVitroSys GmbH. T.T. is the founder and CSO/CMO at Cardior Pharmaceuticals GmbH, a wholly-owned subsidiary of Novo Nordisk Europe A/S. Other authors declare no conflict of interest.
Figures


References
-
- Pearson J, Sipido KR, Musialek P, van Gilst WH. The cardiovascular research community calls for action to address the growing burden of cardiovascular disease. Cardiovasc Res 2019;115:e96–e98. - PubMed
-
- Dowden H, Munro J. Trends in clinical success rates and therapeutic focus. Nat Rev Drug Discov 2019;18:495–496. - PubMed
-
- Ferri N, Siegl P, Corsini A, Herrmann J, Lerman A, Benghozi R. Drug attrition during pre-clinical and clinical development: understanding and managing drug-induced cardiotoxicity. Pharmacol Ther 2013;138:470–484. - PubMed
-
- Weaver RJ, Valentin JP. Today's challenges to De-risk and predict drug safety in human “mind-the-gap”. Toxicol Sci 2019;167:307–321. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

