Mathematical modeling of malaria vaccination with seasonality and immune feedback
- PMID: 40354311
- PMCID: PMC12068631
- DOI: 10.1371/journal.pcbi.1012988
Mathematical modeling of malaria vaccination with seasonality and immune feedback
Abstract
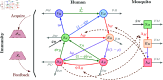
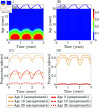
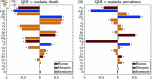
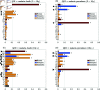
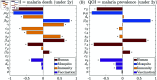
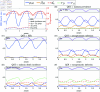
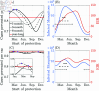
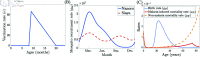
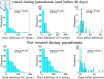
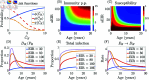
Malaria is one of the deadliest infectious diseases globally, claiming hundreds of thousands of lives each year. The disease presents substantial heterogeneity among the population, with approximately two-thirds of fatalities occurring in children under five years old. Immunity to malaria develops through repeated exposure and plays a crucial role in disease dynamics. Seasonal environmental fluctuations, such as changes in temperature and rainfall, lead to temporal heterogeneity and further complicate transmission dynamics and the utility of intervention strategies. We employ an age-structured partial differential equation model to characterize seasonal malaria transmission and assess vaccination strategies that vary by timing and duration. Our model integrates vector-host epidemiological dynamics across different age groups and nonlinear feedback between transmission and immunity. We calibrate the model to year-round and seasonal malaria settings and conduct extensive sensitivity analyses for both scenarios to systematically assess which assumptions lead to the most uncertainty. We use time-varying sensitivity indices to identify critical disease parameters during low and high transmission seasons. We further investigate the impact of vaccination and its implementation in the seasonal malaria settings. When implementing a three-dose primary vaccination series, seasonally targeted campaigns can prevent significantly more cases per vaccination than constant year-long programs in regions with strong seasonal variation in transmission. In such scenarios, the optimal vaccination interval aligns with the peak in infected mosquito abundance and precedes the peak in malaria transmission. In contrast, seasonal booster programs may provide limited advantages over year-long vaccination. Additionally, while increasing annual vaccination counts can reduce overall disease incidence, it yields marginal improvements in cases prevented per vaccination.
Copyright: © 2025 Qu et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- World Health Organization. World malaria report 2023; 2023. https://www.who.int/teams/global-malaria-programme/reports/world-malaria...
-
- World Health Organization. Global Health Observatory data repository: Life Tables; 2021. https://apps.who.int/gho/data/view.main.61860?lang=en
-
- Bill & Melinda Gates Foundation. Malaria - Eradication, Prevention, Through Innovation & Data; 2024. https://www.gatesfoundation.org/our-work/programs/global-health/malaria
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

