The CLUE postsurgery VTE risk instrument for abdominal and pelvic surgery: validation of patient risk factor component
- PMID: 40354313
- PMCID: PMC12329283
- DOI: 10.1182/bloodadvances.2024015515
The CLUE postsurgery VTE risk instrument for abdominal and pelvic surgery: validation of patient risk factor component
Abstract
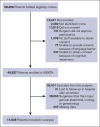
Venous thromboembolism (VTE) remains a major postoperative risk. Systematic reviews have established procedure-specific VTE risk estimates, which form 1 component of the CLUE postsurgery VTE risk instrument. The instrument also incorporates patient-level factors, including age (≥75 years), body mass index (≥35 kg/m2), and prior VTE, to stratify overall risk. However, the patient risk factor component has not been formally validated. Therefore, we conducted the validation using data from the VISION study, a prospective, international cohort of 11 636 patients undergoing major general abdominal, urologic, or gynecologic surgery. Thirty-day postoperative VTE incidence was analyzed using modified Poisson regression. The instrument classified patients into low- (72%), medium- (25%), and high-risk (4%) categories. VTE occurred in 97 patients (0.8%). Compared to the low-risk group, the relative risk of VTE was 1.56 (95% confidence interval [CI], 1.01-2.43) for medium-risk patients and 3.60 (95% CI, 1.90-6.83) for high-risk patients. Among patients who did not receive antithrombotic medication, relative risks increased to 1.91 for medium-risk patients and 5.41 for high-risk patients. The CLUE postsurgery VTE risk instrument, using 3 widely available patient-level factors, accurately classifies patients into substantially different categories of relative VTE risk. This validated patient component complements procedure-specific absolute risk estimates derived from prior systematic reviews. To support evidence-based thromboprophylaxis decisions, the instrument is now available through an interactive online platform (www.cluevte.org).
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: D.S. reported honoraria paid to her institution from AstraZeneca, Bristol Myers Squibb-Pfizer, Roche, and Servier, unrelated to the current work. P.J.D. reported receiving grants from Abbott Diagnostics, AOP Pharma, Renibus, Roche Diagnostics, and Siemens; monitoring services from CloudDX and Philips Healthcare; serving as a consultant for Abbott Diagnostics, AstraZeneca, Bayer, Roche Canada, and Trimedic; and serving as an advisory board member for Bayer and Quidel outside the submitted work. F.K.B. reported receiving investigator-initiated grants from Roche Diagnostics and Siemens, unrelated to the current work. K.A.O.T. was chair of the European Association of Urology Guideline on Thromboprophylaxis in Urological Surgery; a panel member of the American Society of Hematology Thromboprophylaxis guideline on prevention of venous thromboembolism (VTE) in surgical hospitalized patients; and urology subgroup chair of the European Society of Anesthesiology and Intensive Care Task Force for the European Guidelines on VTE. G.H.G. was a panel member of the European Association of Urology Guideline on Thromboprophylaxis in Urological Surgery. The remaining authors declare no competing financial interests.
A complete list of collaborator authors of CLUE Postsurgery VTE Risk Instrument Group appears in “Appendix.”
Figures


References
-
- Meara JG, Leather AJ, Hagander L, et al. Global Surgery 2030: evidence and solutions for achieving health, welfare, and economic development. Lancet. 2015;386(9993):569–624. - PubMed
-
- Devereaux PJ, Sessler DI. Cardiac complications in patients undergoing major noncardiac surgery. N Engl J Med. 2015;373(23):2258–2269. - PubMed
-
- Tikkinen KAO, Cartwright R, Gould MK, et al. Thromboprophylaxis in urological surgery. https://d56bochluxqnz.cloudfront.net/documents/full-guideline/EAU-Guidel...
-
- National Institute for Health and Care Excellence Venous thromboembolism in over 16s: reducing the risk of hospital-acquired deep vein thrombosis or pulmonary embolism. NICE guideline NG89. https://www.nice.org.uk/guidance/ng89 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

