Dehomologative C-C Borylation of Aldehydes and Alcohols via a Rh-Catalyzed Dehydroformylation-Borylation Relay
- PMID: 40354369
- PMCID: PMC12100658
- DOI: 10.1021/jacs.5c02181
Dehomologative C-C Borylation of Aldehydes and Alcohols via a Rh-Catalyzed Dehydroformylation-Borylation Relay
Abstract
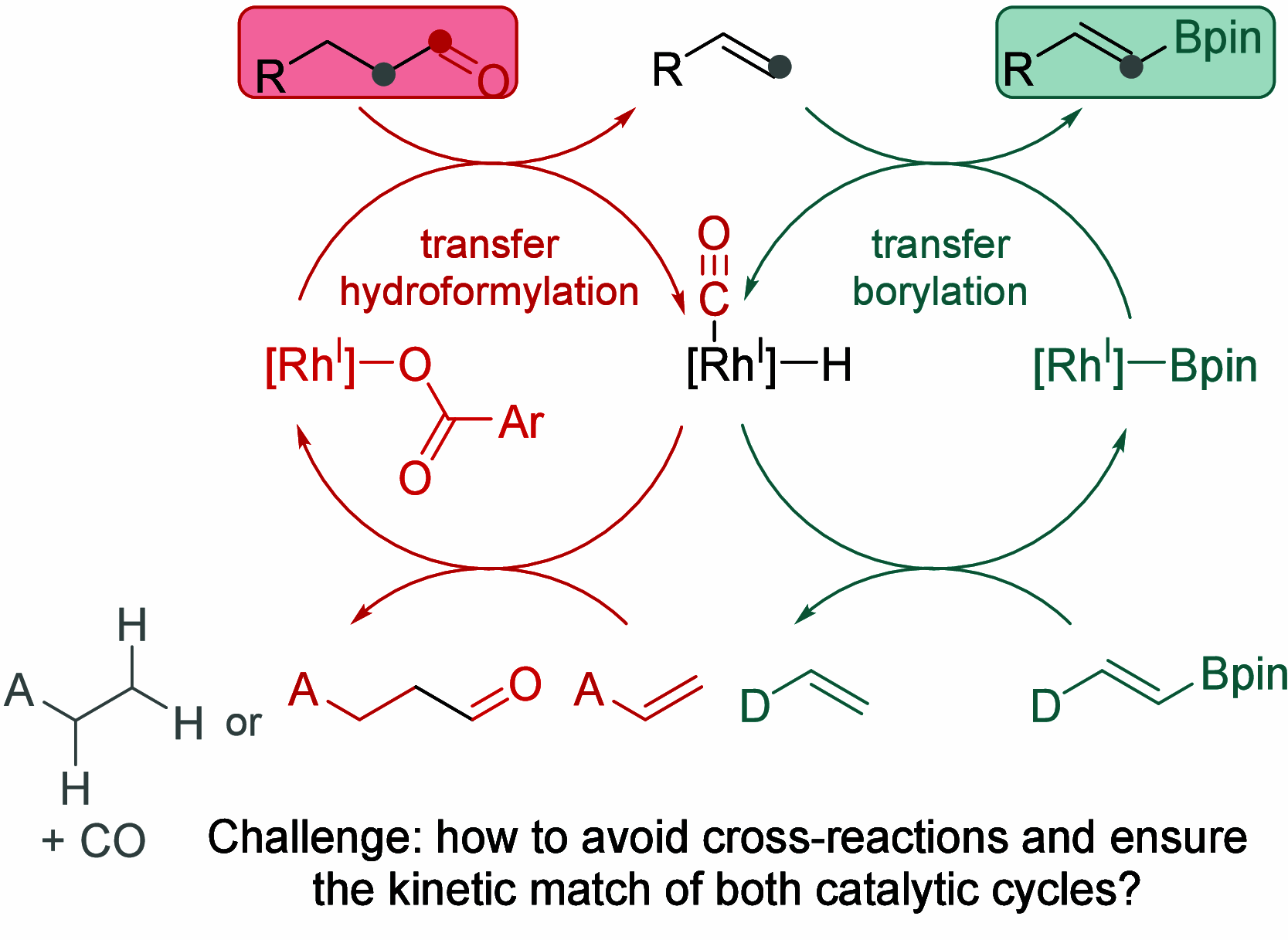
The dehomologative conversion of linear or α-methyl aldehydes to vinyl boronates is achieved via a one-pot sequence of rhodium-catalyzed transfer dehydroformylation and transfer borylation of the resulting alkenes. Similarly, allylic or aliphatic alcohols are converted to vinyl boronates through a sequence involving, respectively, rhodium-catalyzed isomerization or transfer dehydrogenation to aldehyde intermediates, followed by dehydroformylation-borylation. The vinyl boronates can be further hydrogenated to alkyl boronates using the same rhodium precatalyst, enabling all five catalytic steps with a single catalyst system.
Figures





Similar articles
-
Functionalization of unactivated alkenes through iridium-catalyzed borylation of carbon-hydrogen bonds. Mechanism and synthetic applications.J Org Chem. 2009 Oct 16;74(20):7715-23. doi: 10.1021/jo9014694. J Org Chem. 2009. PMID: 19775089
-
Alpha-hydroxyalkyl heterocycles via chiral allylic boronates: Pd-catalyzed borylation leading to a formal enantioselective isomerization of allylic ether and amine.J Am Chem Soc. 2009 Jul 22;131(28):9612-3. doi: 10.1021/ja903946f. J Am Chem Soc. 2009. PMID: 19552416
-
Rhodium-Catalyzed Dehydrogenative Borylation of Aliphatic Terminal Alkenes with Pinacolborane.Angew Chem Int Ed Engl. 2015 Oct 19;54(43):12659-63. doi: 10.1002/anie.201506328. Epub 2015 Sep 2. Angew Chem Int Ed Engl. 2015. PMID: 26332866
-
Nickel-Catalyzed Homo- and Cross-Coupling of Allyl Alcohols via Allyl Boronates.Org Lett. 2020 Jun 5;22(11):4418-4423. doi: 10.1021/acs.orglett.0c01424. Epub 2020 May 19. Org Lett. 2020. PMID: 32427489
-
Synthesis of Quinolines via a Metal-Catalyzed Dehydrogenative N-Heterocyclization.Chem Rec. 2017 Feb;17(2):200-216. doi: 10.1002/tcr.201600083. Epub 2016 Aug 15. Chem Rec. 2017. PMID: 27524555 Review.
References
-
-
For selected reviews and examples of studies on the synthesis and functionalisation of aldehydes and alcohols, see:
- Mandal T., Mallick S., Islam M., De Sarkar S.. Alcohols as Alkyl Synthons Enabled by Photoredox-Catalyzed Deoxygenative Activation. ACS Catal. 2024;14(17):13451–13496. doi: 10.1021/acscatal.4c03560. - DOI
- Villo P., Shatskiy A., Kärkäs M. D., Lundberg H.. Electrosynthetic C–O Bond Activation in Alcohols and Alcohol Derivatives. Angew. Chem., Int. Ed. 2023;62(4):e202211952. doi: 10.1002/anie.202211952. - DOI - PMC - PubMed
- Estopiñá-Durán S., Taylor J. E.. Brønsted Acid-Catalysed Dehydrative Substitution Reactions of Alcohols. Chem. – Eur. J. 2021;27(1):106–120. doi: 10.1002/chem.202002106. - DOI - PMC - PubMed
- Lainer B., Das K., Dydio P.. Variable Structure Diversification by Multicatalysis: The Case of Alcohols. Chem. Commun. 2023;59(32):4716–4725. doi: 10.1039/D3CC00551H. - DOI - PMC - PubMed
- Li J., Huang C., Li C.. Deoxygenative Functionalizations of Aldehydes, Ketones and Carboxylic Acids. Angew. Chem., Int. Ed. 2022;61(10):e202112770. doi: 10.1002/anie.202112770. - DOI - PubMed
- Spinello B. J., Strong Z. H., Ortiz E., Evarts M. M., Krische M. J.. Intermolecular Metal-Catalyzed C–C Coupling of Unactivated Alcohols or Aldehydes for Convergent Ketone Construction beyond Premetalated Reagents. ACS Catal. 2023;13(16):10976–10987. doi: 10.1021/acscatal.3c02209. - DOI - PMC - PubMed
- Amistadi-Revol H., Liu S., Prévost S.. C–H Functionalization of Aldehydes and Ketones with Transient Directing Groups: Recent Developments. Eur. J. Org. Chem. 2023;26(31):e202300582. doi: 10.1002/ejoc.202300582. - DOI
- Zheng Y.-L., Newman S. G.. Cross-Coupling Reactions with Esters, Aldehydes, and Alcohols. Chem. Commun. 2021;57(21):2591–2604. doi: 10.1039/D0CC08389E. - DOI - PubMed
- Davison R. T., Kuker E. L., Dong V. M.. Teaching Aldehydes New Tricks Using Rhodium- and Cobalt-Hydride Catalysis. Acc. Chem. Res. 2021;54(5):1236–1250. doi: 10.1021/acs.accounts.0c00771. - DOI - PMC - PubMed
- Beddoe R. H., Andrews K. G., Magné V., Cuthbertson J. D., Saska J., Shannon-Little A. L., Shanahan S. E., Sneddon H. F., Denton R. M.. Redox-Neutral Organocatalytic Mitsunobu Reactions. Science. 2019;365(6456):910–914. doi: 10.1126/science.aax3353. - DOI - PubMed
- Hill C. K., Hartwig J. F.. Site-Selective Oxidation, Amination and Epimerization Reactions of Complex Polyols Enabled by Transfer Hydrogenation. Nat. Chem. 2017;9(12):1213–1221. doi: 10.1038/nchem.2835. - DOI - PMC - PubMed
- Simmons E. M., Hartwig J. F.. Catalytic Functionalization of Unactivated Primary C–H Bonds Directed by an Alcohol. Nature. 2012;483(7387):70–73. doi: 10.1038/nature10785. - DOI - PMC - PubMed
- Chen R., Intermaggio N. E., Xie J., Rossi-Ashton J. A., Gould C. A., Martin R. T., Alcázar J., MacMillan D. W. C.. Alcohol-Alcohol Cross-Coupling Enabled by SH 2 Radical Sorting. Science. 2024;383(6689):1350–1357. doi: 10.1126/science.adl5890. - DOI - PMC - PubMed
- Pirnot M. T., Rankic D. A., Martin D. B. C., MacMillan D. W. C.. Photoredox Activation for the Direct Beta-Arylation of Ketones and Aldehydes. Science. 2013;339(6127):1593–1596. doi: 10.1126/science.1232993. - DOI - PMC - PubMed
- Wang J. Z., Lyon W. L., MacMillan D. W. C.. Alkene Dialkylation by Triple Radical Sorting. Nature. 2024;628(8006):104–109. doi: 10.1038/s41586-024-07165-x. - DOI - PMC - PubMed
-
-
- Homologation Reactions: Reagents, Applications and Mechanisms; Pace, V. , Ed.; Wiley-VCH: Weinheim, Germany, 2023.
LinkOut - more resources
Full Text Sources

