Effect of PGPR on growth and nutrient utilization of Elymus nutans Griseb at different temperatures
- PMID: 40354404
- PMCID: PMC12068589
- DOI: 10.1371/journal.pone.0323613
Effect of PGPR on growth and nutrient utilization of Elymus nutans Griseb at different temperatures
Abstract
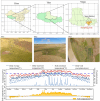
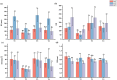
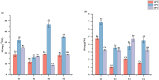
Plant growth-promoting rhizobacteria (PGPR) are beneficial bacteria that facilitate plant growth and can be used in the restoration of ecosystems. However, PGPR vary in their temperature tolerance, and few studies have investigated the effect of temperature on PGPR-mediated growth promotion or PGPR inoculum colonization. Therefore, we isolated and purified rhizosphere bacteria from the rhizosphere soil of Elymus nutans Griseb (EnG), collected from the Qinghai-Tibet Plateau. Selective culture media were used to assess whether these strains possess plant growth-promoting abilities and to measure the magnitude of their plant growth-promoting ability. Then screen out the strains (S1, S2, S3, S4, and S5) with strong plant growth-promoting ability for identification. To demonstrate the growth-promoting effects of the selected PGPR, we conducted a study. In this study, we simulated three temperature gradients (10°C, 15°C, and 20°C) during the growing season of EnG on the Tibetan Plateau. Furthermore, we established four incubation substrate treatments: T1(addition of PGPR but no addition of NPK fertilizers), T2 (neither PGPR nor NPK fertilizers addition), T3 (addition of PGPR both and NPK fertilizers), and T4 (addition of NPK fertilizers but not PGPR), to explore the effects of PGPR on the growth and nutrient (NPK) utilization efficiency of EnG at different temperatures. The results revealed that compared with those under T2, the plant height (PT) and dry weight under, T1 increased by 51.72% - 70.67% and 24.99-51.25%, respectively. The soluble sugar (SS) and soluble protein (SP) content significantly increased by 59.37% and 369.66%, respctively, at 10 °C (p < 0.05) and by 100.17% and 94.5%, respectively, at 15 °C (p < 0.05). Compared with those under T4, the physiological efficiencies of N (NPE) at 15 °C and 20 °C significantly decreased by 40.43% and 72.11%, respectively, under T3. In summary, these showed that this PGPR (S1, S2, S3, S4, and S5) promoted the growth of EnG on the Tibetan plateau and improved its nutrient utilization efficiency.
Copyright: © 2025 Ran et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Fan X, Zhang S, Mo X, Li Y, Fu Y, Liu Z. Effects of Plant Growth-Promoting Rhizobacteria and N Source on Plant Growth and N and P Uptake by Tomato Grown on Calcareous Soils. Pedosphere. 2017;27(6):1027–36. doi: 10.1016/s1002-0160(17)60379-5 - DOI
-
- Vessey JK. Plant growth promoting rhizobacteria as biofertilizers. Plant and Soil. 2003;255(2):571–86. doi: 10.1023/a:1026037216893 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

