Isoprenaline shows unique kinase dependencies in stimulating β1AR-β-arrestin2 interaction compared to endogenous catecholamines
- PMID: 40354729
- PMCID: PMC12264555
- DOI: 10.1016/j.molpha.2025.100041
Isoprenaline shows unique kinase dependencies in stimulating β1AR-β-arrestin2 interaction compared to endogenous catecholamines
Abstract
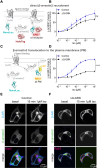
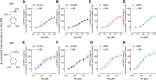
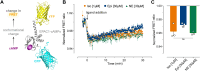
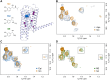
The β1-adrenergic receptor (β1AR) is an essential G protein-coupled receptor in the heart. Its dysregulation represents a hallmark of cardiac diseases. Studies have identified a unique mode of β-arrestin interaction, where β1AR briefly engages with β-arrestins before catalytically accumulating them at the plasma membrane (PM) independently of the receptor. Although receptor phosphorylation crucially impacts β-arrestins, the contributions of specific kinases vital in β1AR regulation remain unclear. Here, we employed G protein-coupled receptor kinase (GRK) GRK2/3/5/6 knockout cells and the protein kinase A inhibitor H89 in bioluminescence resonance energy transfer-based assays to systematically assess GRKs and protein kinase A in direct β-arrestin2 recruitment to β1AR and β-arrestin2 translocation to the PM. Furthermore, we compared the effects of the synthetic agonist isoprenaline with the endogenous catecholamines: epinephrine and norepinephrine. We observed pronounced differences in their kinase dependencies to mediate β-arrestin2 translocation to the PM. Upon isoprenaline stimulation, GRKs strongly influenced β-arrestin2 translocation to the PM but had no effect on direct β-arrestin2 recruitment to β1AR. Additionally, in a GRK2-specific context, protein kinase A inhibition primarily reduced the efficacy of isoprenaline for β-arrestin2 translocation, whereas for GRK5, it decreased potency. Strikingly, these kinase-dependent effects were absent for epinephrine and norepinephrine, suggesting distinct underlying molecular mechanisms for β-arrestin2 accumulation at the PM. This observation could be explained by agonist-specific differences in receptor conformational rearrangements, as suggested by distinct changes in the NMR spectra of β1AR. Our findings highlight that synthetic and endogenous ligands induce distinct molecular mechanisms in β1AR regulation, emphasizing the need to consider these differences when translating molecular insights into physiological contexts. SIGNIFICANCE STATEMENT: Our findings reveal mechanistic differences in β1-adrenergic receptor-mediated catalytic activation of β-arrestin2 by synthetic and endogenous agonists, driven by distinct G protein-coupled receptor kinases and protein kinase A dependencies. Although β-arrestin2 translocation to the PM occurred to similar extents with isoprenaline, epinephrine, and norepinephrine, kinase involvement was crucial only upon Iso stimulation of β1-adrenergic receptor. By elucidating these ligand-specific pathways, this study advances our understanding of β1-adrenergic receptor signaling and regulation while additionally highlighting the importance of considering these differences when translating molecular insights into pathophysiological contexts.
Keywords: Bioluminescence resonance energy transfer; G protein-coupled receptor kinases; G protein-coupled receptors; Nuclear magnetic resonance spectroscopy; Protein kinase A; β-arrestin2 translocation.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest Carsten Hoffmann reports financial support was provided by European Regional Development Fund. Alvar D. Gossert reports financial support was provided by Swiss National Science Foundation. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Benovic J.L., Kühn H., Weyand I., Codina J., Caron M.G., Lefkowitz R.J. Functional desensitization of the isolated beta-adrenergic receptor by the beta-adrenergic receptor kinase: potential role of an analog of the retinal protein arrestin (48-kDa protein) Proc Natl Acad Sci U S A. 1987;84:8879–8882. - PMC - PubMed
-
- Boucrot E., Ferreira A.P., Almeida-Souza L., Debard S., Vallis Y., Howard G., Bertot L., Sauvonnet N., McMahon H.T. Endophilin marks and controls a clathrin-independent endocytic pathway. Nature. 2015;517:460–465. - PubMed
-
- Bristow M.R., Ginsburg R., Umans V., Fowler M., Minobe W., Rasmussen R., Zera P., Menlove R., Shah P., Jamieson S., et al. Beta 1- and beta 2-adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: coupling of both receptor subtypes to muscle contraction and selective beta 1-receptor down-regulation in heart failure. Circ Res. 1986;59:297–309. - PubMed
-
- Brodde O.E. Beta-adrenoceptors in cardiac disease. Pharmacol Ther. 1993;60:405–430. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

