Pharmacokinetics and Pharmacodynamics of Remimazolam for Procedural Sedation in Children and Adolescents
- PMID: 40355106
- PMCID: PMC12227205
- DOI: 10.1097/ALN.0000000000005560
Pharmacokinetics and Pharmacodynamics of Remimazolam for Procedural Sedation in Children and Adolescents
Abstract
Background: Remimazolam is not approved for use in pediatric patients. The pharmacokinetics of remimazolam have been reported to be similar to those of adult patients after scaling for body size. This article reports on the pharmacokinetics and pharmacodynamics of pediatric patients aged 6 to 18 yr and a subsequent model-based optimization of the used dosing regimen.
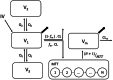
Methods: Thirty-one patients were included in the trial and stratified across four treatment arms: bolus administration, infusion, bolus plus fentanyl, or infusion plus fentanyl. The University of Michigan (Ann Arbor, Michigan) Sedation Scale (UMSS) was used to assess the depth of sedation. Blood samples were drawn to measure the concentrations of remimazolam and its metabolite CNS7054. Population pharmacokinetic pharmacodynamic modeling was performed in NONMEM (GloboMax LLC, USA).
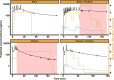
Results: A population pharmacokinetic model was developed for remimazolam and CNS7054. The elimination clearance of remimazolam was 0.70 l · min -1 · 70 kg -1 . A proportional odds model combined with a simplified Minto model described the observed UMSS well. The EC50 of remimazolam for a UMSS score of 3 or greater was 777 ng · ml -1 in the absence of fentanyl, and decreased to 655, 533, and 287 ng/ml for concomitant fentanyl steady state concentrations of 1, 2, or 4 ng · ml -1 , respectively. Simulations confirmed that the studied dosing regimen resulted in 9.2 to 22.0% of patients not reaching a UMSS score of 3 or greater at the end of the induction. Model-based optimization resulted in higher per-kilogram dosages and the removal of the maximum allowable dose. Simulations indicated that the percentage of patients achieving a UMSS score of 3 or greater can be expected to be high (88 to 97%).
Conclusions: This study has shown that the pharmacokinetics of remimazolam are likely different between children 6 yr or older and adults (after correcting for size). In addition, the exposure-response relationship shows that to effectively use remimazolam for procedural sedation in children 6 yr or older, the dosing regimen should be modified to allow for higher remimazolam exposures.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Society of Anesthesiologists.
Conflict of interest statement
Dr. Struys declares that his research group/department received (during the last 3 yr) research grants and consultancy fees from The Medicines Company (Parsippany, New Jersey), Masimo (Irvine, California), Becton Dickinson (Eysins, Switzerland), Fresenius (Bad Homburg, Germany), Dräger (Lübeck, Germany), Paion (Aachen, Germany), and Medtronic (Dublin, Ireland). He receives royalties on intellectual property from Demed Medical (Temse, Belgium) and Ghent University (Ghent, Belgium). He is an editorial board member and director for the
The article processing charge was funded by Groningen University.
Figures


Similar articles
-
Development and analysis of a remimazolam pharmacokinetics and pharmacodynamics model with proposed dosing and concentrations for anaesthesia and sedation.Br J Anaesth. 2025 Jul;135(1):206-217. doi: 10.1016/j.bja.2025.02.038. Epub 2025 Apr 30. Br J Anaesth. 2025. PMID: 40312166
-
Pharmacokinetic Properties and Therapeutic Effectiveness of Remimazolam in ICU Patients With Mechanical Ventilation: A Preliminary Study.Pharmacol Res Perspect. 2025 Jun;13(3):e70130. doi: 10.1002/prp2.70130. Pharmacol Res Perspect. 2025. PMID: 40517312 Free PMC article. Clinical Trial.
-
Pharmacokinetic and pharmacodynamic characterization of remimazolam in older Japanese adults who underwent general anesthesia at early stage of infusion.Sci Rep. 2025 Jul 10;15(1):24979. doi: 10.1038/s41598-025-10015-z. Sci Rep. 2025. PMID: 40640376 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Remimazolam for procedural sedation: A systematic review with meta-analyses and trial sequential analyses.Eur J Anaesthesiol. 2025 Apr 1;42(4):298-312. doi: 10.1097/EJA.0000000000002126. Epub 2025 Feb 4. Eur J Anaesthesiol. 2025. PMID: 39905818
References
-
- Committee for Medicinal Products for Human Use (CHMP): Byfavo - Assessment Report (EMEA/H/C/005246/0000). 2021. Available at: https://www.ema.europa.eu/en/documents/assessmentreport/byfavo-epar-publ.... Accessed June 12, 2025.
-
- FDA: BYFAVO™ (remimazolam) for injection, for intravenous use. 2020. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212295s000lbl.pdf. Accessed June 12, 2025.
-
- Gao YQ, Ihmsen H, Hu ZY, et al.: Pharmacokinetics of remimazolam after intravenous infusion in anaesthetised children. Br J Anaesth 2023; 131:914–20. doi:10.1016/j.bja.2023.08.019 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

