Long-term mortality outcomes among immunotherapy recipients treated with dupilumab for the management of cutaneous immune-related adverse events
- PMID: 40355282
- PMCID: PMC12083386
- DOI: 10.1136/jitc-2024-010638
Long-term mortality outcomes among immunotherapy recipients treated with dupilumab for the management of cutaneous immune-related adverse events
Abstract
Background: Dupilumab has been added to National Cancer Comprehensive Network guidelines as a therapeutic strategy for managing certain cutaneous immune-related adverse events (cirAEs) from immune checkpoint blockade (ICB). However, little is known about the implications of dupilumab for cancer outcomes in this population. In this multi-institutional study, we evaluate the impact of dupilumab treatment on survival among ICB recipients.
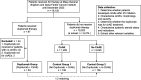
Methods: We conducted a multi-institutional retrospective cohort study of ICB recipients from the Mass General Brigham Healthcare System and Dana-Farber Cancer Institute. The dupilumab group was compared with two control groups who did not receive dupilumab: with and without cirAEs (control groups 1 and 2, respectively) that were 1:2 matched on sex, race, age at ICB initiation, Charlson Comorbidity Score, year of ICB initiation, and ICB type. Manual chart review was performed to obtain cirAE characteristics, systemic glucocorticoid use, dupilumab treatment, vital status, and last contact date. Time-varying multivariable Cox proportional hazards regressions were used to evaluate the impact of dupilumab on overall survival, adjusted for sex, race, age at ICB initiation, ICB type, Charlson Comorbidity Index score, cancer type, cancer stage at ICB initiation, and systemic glucocorticoid use.
Results: A total of 53 cirAE patients treated with dupilumab were compared with two control groups of 106 patients each. Most patients receiving dupilumab demonstrated either complete or partial resolution of their cirAE (88.7%). In multivariable modeling, the overall survival of the dupilumab group was not significantly different from control group 1 (HR=0.74, 95% CI: 0.35 to 1.60, p=0.5) or control group 2 (HR=0.70, 95% CI: 0.32 to 1.51, p=0.4). However, the use of systemic glucocorticoids within 2 years after ICB initiation was associated with poorer overall survival when comparing the dupilumab group to control group 1 (HR=2.03, 95% CI: 1.04 to 3.96, p=0.039) and control group 2 (HR=2.21, 95% CI: 1.25 to 3.91, p=0.006).
Conclusions: This study suggests that dupilumab is an effective therapy for recalcitrant cirAEs and does not adversely impact mortality. Due to the observed detrimental effects of systemic glucocorticoid therapy, this study suggests the need to shift away from systemic glucocorticoid immunosuppression and toward targeted immune modulators for irAE management, though prospective randomized trials are necessary to investigate this.
Keywords: Immune Checkpoint Inhibitor; Immune modulatory; Immune related adverse event - irAE; Immunotherapy; Rash.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: YRS is an advisory board member or consultant and has received honoraria from Pfizer, Incyte Corporation, Sanofi, Galderma, Castle Biosciences, and Iovance Biotherapeutics. KLR is an advisory board member to SAGA Diagnostics, has received speaker fees from CMEOutfitters, MedScape, and BMS, and provides institutional support for the ATRIUM clinical trial. NRL is a consultant and has received honoraria from Bayer, Silverback, Fortress Biotech, and Synox Therapeutics outside the scope of the submitted work.
Figures
Update of
-
Long-term mortality outcomes among immunotherapy recipients treated with dupilumab for the management of cutaneous immune-related adverse events.medRxiv [Preprint]. 2025 Jan 8:2025.01.07.25320156. doi: 10.1101/2025.01.07.25320156. medRxiv. 2025. Update in: J Immunother Cancer. 2025 May 12;13(5):e010638. doi: 10.1136/jitc-2024-010638. PMID: 39830283 Free PMC article. Updated. Preprint.
References
-
- [18-Jun-2024]. https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf Available. Accessed.
-
- Horvat TZ, Adel NG, Dang T-O, et al. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol. 2015;33:3193–8. doi: 10.1200/JCO.2015.60.8448. - DOI - PMC - PubMed
-
- Riudavets M, Mosquera J, Garcia-Campelo R, et al. Immune-Related Adverse Events and Corticosteroid Use for Cancer-Related Symptoms Are Associated With Efficacy in Patients With Non-small Cell Lung Cancer Receiving Anti-PD-(L)1 Blockade Agents. Front Oncol. 2020;10:1677. doi: 10.3389/fonc.2020.01677. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
