Associations between immune checkpoint inhibitor response, immune-related adverse events, and steroid use in RADIOHEAD: a prospective pan-tumor cohort study
- PMID: 40355283
- PMCID: PMC12083316
- DOI: 10.1136/jitc-2025-011545
Associations between immune checkpoint inhibitor response, immune-related adverse events, and steroid use in RADIOHEAD: a prospective pan-tumor cohort study
Abstract
Background: Immune checkpoint inhibitors (ICIs) have led to enduring responses in subsets of patients with cancer. However, these responses carry the risk of immune-related adverse events (irAEs), which can diminish the overall benefit of ICI treatment. While associations between irAE development and overall survival have been increasingly documented, there is a need for further understanding of these connections in large prospective real-world cohorts.
Methods: The Resistance Drivers for Immuno-Oncology Patients Interrogated by Harmonized Molecular Datasets (RADIOHEAD) study, a pan-tumor, prospective cohort of 1,070 individuals undergoing standard of care first-line ICI treatment, aims to identify factors driving irAEs and clinical response. Clinical data and longitudinal blood samples were collected prospectively at multiple time points from 49 community-based oncology clinics across the USA. Structured, harmonized clinical data underwent unbiased statistical analysis to uncover predictors of real-world overall survival (rwOS) and risk factors for irAEs.
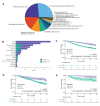
Results: Across 1,070 participants' treatment courses, RADIOHEAD accumulated over 4,500 clinical data points. Patients experiencing any irAE (25.4%, n=272) exhibited significantly improved rwOS in the pan-tumor cohort (n=1,028, HR=0.41, 95% CI=(0.31, 0.55)). This association persisted when adjusting for age and metastatic disease in multivariate time-dependent Cox proportional hazard analysis, and was consistent across major tumor subtypes, including lung cancer and melanoma. Skin and endocrine irAEs of any grade were strongly associated with improved rwOS (Cox proportional hazard analysis, skin, p=2.03e-05; endocrine, p=0.0006). In this real-world cohort, the irAE rate appeared lower than those reported in clinical trials. Patients receiving corticosteroids prior to initiation of ICI treatment had significantly worse survival outcomes than non-users (HR 1.37, p=0.0054), with a stronger association with systemic steroid use (HR 1.75, p=0.0022). The risk of irAE was increased by exposure to combination immunotherapy relative to monotherapy (OR 4.17, p=2.8e-7), zoster vaccine (OR 2.4, p=5.2e-05), and decreased by prior chemotherapy (OR 1.69, p=0.0005).
Conclusion: The RADIOHEAD cohort is a well-powered, real-world cohort that clearly demonstrates the association between irAE development with improved response and baseline steroid use with worse response to ICI treatment after adjustment for survival bias.
Keywords: Immune Checkpoint Inhibitors; Immune related adverse event - irAE; Immunotherapy.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: ZQ has consulted for Sanofi and Novartis. MSA owns stock in Merck and Medtronic. SIL owns stock in AbbVie. LHB has served on advisory boards for Calidi Biotherapeutics, Western Oncolytics/Kalivir, Torque Therapeutics/Repertoire, Pyxis, CytomX, Roche-Genentech (Biomarkers Roundtable), Khloris, Takeda Oncology, Adaptimmune, RAPT, and DCPrime. LHB holds stocks or shares in Repertoire, Pyxis, RAPT, and CytomX.
Figures


References
-
- Horvat TZ, Adel NG, Dang T-O, et al. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol. 2015;33:3193–8. doi: 10.1200/JCO.2015.60.8448. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
