Effect of histidine and carnosine on haemoglobin recovery in anaemia induced-kidney damage and iron-loading mouse models
- PMID: 40355605
- PMCID: PMC12069506
- DOI: 10.1007/s00726-025-03451-8
Effect of histidine and carnosine on haemoglobin recovery in anaemia induced-kidney damage and iron-loading mouse models
Abstract
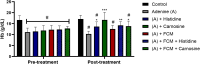
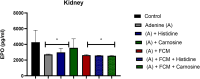
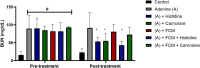
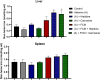
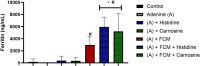
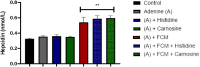
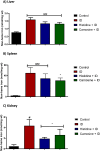
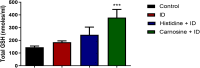
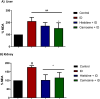
Histidine and carnosine can form complexes with divalent metal ions such as Fe2+, potentially providing stability to intracellular labile iron. Anaemia is a common comorbidity in the late stages of kidney disease, and patients are treated with erythropoiesis-stimulating agents (ESAs) and iron supplementation. However, iron supplementation is also associated with worse long-term outcomes. The purpose of this study is to investigate how histidine and carnosine supplementation can reduce symptoms of anaemia of chronic kidney disease (CKD) and the effects associated with iron-overloaded conditions. Adenine-induced chronic kidney disease mice were treated with histidine and carnosine by oral gavage for 10 days. Additionally, a model involving iron overload in mice was established, and these mice received concurrent treatment with histidine and carnosine. Haemoglobin, non-haem iron, malondialdehyde (MDA) and iron parameters were measured. Carnosine increased erythropoietin (EPO) levels (35.62 µg/ml ± 11.43) and resulted in haemoglobin repletion (16.7 g/dL ± 3.4). When iron was supplemented alongside with histidine or carnosine, there were better effects on haemoglobin repletion (14.22 ± 1.7 and 13.82 ± 2.15 g/ dL respectively), ferritin (59.5 ± 16.4, 52 ± 29.5 µg/ml) and non-haem iron (0.8 ± 0.21, 0.7 ± 0.38 nmol/mg), than the group receiving iron alone (p < 0.05). Furthermore, histidine and carnosine reduced non-haem iron and MDA, in iron-loaded conditions (p < 0.05). These positive effects observed in histidine and carnosine could be associated with reactive oxygen species (ROS) scavenging. EPO restoring levels in CKD model and the increment in haemoglobin and ferritin in carnosine treatments suggested the potential formation of a ternary complex with iron-glutathione. In conclusion, our results indicate the beneficial effect of histidine and carnosine in the context of iron supplementation for the correction of haemoglobin and protection against iron-loaded conditions.
Keywords: Adenine; Anaemia; Carnosine; Disease; Histidine; Kidney.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

References
-
- Atkinson MA, White CT (2012) ‘Hepcidin in anemia of chronic kidney disease: Review for the pediatric nephrologist’, Pediatric Nephrology, pp. 33–40. 10.1007/s00467-011-1832-y - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

