Nrf2 deficiency enhances oxidative stress and promotes susceptibility to tinnitus in mice
- PMID: 40355648
- PMCID: PMC12069615
- DOI: 10.1038/s41598-025-01509-x
Nrf2 deficiency enhances oxidative stress and promotes susceptibility to tinnitus in mice
Abstract
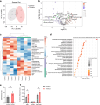
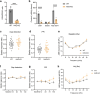
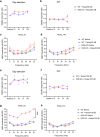
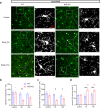
Tinnitus is a prevalent and distressing medical symptom, and no effective pharmacological treatment currently exists. Despite significant advances, tinnitus remains a scientific enigma. To explore the molecular underpinnings of tinnitus, we developed a noise-induced tinnitus model in mice and utilized metabolomics to identify key differences in metabolic pathways. Our results revealed that oxidative stress-related pathways, including glutathione (GSH) metabolism, were significantly enriched in the auditory cortex of mice exhibiting tinnitus-like behavior. To further explore the role of oxidative stress, we examined the involvement of nuclear factor erythroid 2-related factor 2 (Nrf2) in tinnitus by conducting experiments in Nrf2 knockout (Nrf2-KO) mice. While Nrf2-deficient mice did not develop spontaneous tinnitus or hearing loss, they displayed increased susceptibility to prolonged tinnitus-like behavior after noise exposure. This was accompanied by heightened microglial activation, neuroinflammation, and significant alterations in gut microbiota composition, including greater diversity and dysbiosis. Our findings highlight a novel mechanism underlying tinnitus, emphasizing the role of oxidative stress in the auditory cortex and its connection to noise-induced tinnitus. The deficiency of Nrf2 in mice increases their susceptibility to tinnitus, suggesting that Nrf2 may serve as a promising therapeutic target for preventing noise-induced tinnitus.
Keywords: Glutathione; Gut microbiota dysbiosis; Nrf2; Oxidative stress; Tinnitus.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

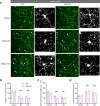
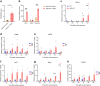
Similar articles
-
NRF2 Is a Key Target for Prevention of Noise-Induced Hearing Loss by Reducing Oxidative Damage of Cochlea.Sci Rep. 2016 Jan 18;6:19329. doi: 10.1038/srep19329. Sci Rep. 2016. PMID: 26776972 Free PMC article.
-
Nrf2 deficiency induces oxidative stress and promotes RANKL-induced osteoclast differentiation.Free Radic Biol Med. 2013 Dec;65:789-799. doi: 10.1016/j.freeradbiomed.2013.08.005. Epub 2013 Aug 14. Free Radic Biol Med. 2013. PMID: 23954472
-
The role of nuclear factor E2-Related factor 2 and uncoupling protein 2 in glutathione metabolism: Evidence from an in vivo gene knockout study.Biochem Biophys Res Commun. 2016 Sep 9;478(1):87-92. doi: 10.1016/j.bbrc.2016.07.088. Epub 2016 Jul 21. Biochem Biophys Res Commun. 2016. PMID: 27453341
-
Neuroinflammation mediates noise-induced synaptic imbalance and tinnitus in rodent models.PLoS Biol. 2019 Jun 18;17(6):e3000307. doi: 10.1371/journal.pbio.3000307. eCollection 2019 Jun. PLoS Biol. 2019. PMID: 31211773 Free PMC article.
-
Auditory thalamic circuits and GABAA receptor function: Putative mechanisms in tinnitus pathology.Hear Res. 2017 Jun;349:197-207. doi: 10.1016/j.heares.2016.08.009. Epub 2016 Aug 21. Hear Res. 2017. PMID: 27553899 Free PMC article. Review.
References
-
- Mølhave, M., Udholm, S., Hawton, K., Ovesen, T. & Erlangsen, A. Association between hospital-diagnosed tinnitus and suicide: A nationwide Danish longitudinal study. J. Psychosom. Res.185, 111879 (2024). - PubMed
-
- Baguley, D., McFerran, D., Hall, D. & Tinnitus Lancet382, 1600–1607 (2013). - PubMed
-
- Lockwood, A. H., Salvi, R. J., Burkard, R. F. & Tinnitus N Engl. J. Med.347, 904–910 (2002). - PubMed
MeSH terms
Substances
Grants and funding
- YCJJ20242120/Fundamental Research Funds for the Central Universities
- 2024BRA019/Fundamental Research Funds for the Central Universities
- 2023AFA038/Foundation for Innovative Research Groups of Hubei Province
- 2021YFF0702303/National Key Research and Development Program of China
- 82430035/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

