FOSL1 promotes keratinocyte migration and wound repair by modulating the IL17 signaling pathway
- PMID: 40355666
- PMCID: PMC12069625
- DOI: 10.1038/s41598-025-99128-z
FOSL1 promotes keratinocyte migration and wound repair by modulating the IL17 signaling pathway
Abstract
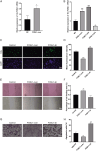
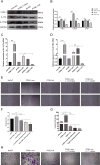
Keratinocytes, the most important cell type constituting the epidermis, migrate to restore the epithelial barrier during wound healing and are a crucial step in wound healing. This study utilized bioinformatics analysis of comprehensive expression datasets of aberrantly expressed genes in wound healing to identify the abnormal expression of the critical transcription factor Fos-like antigen-1 (FOSL1), which is involved in various diseases. Currently, there is limited research on the role of FOSL1 in wound healing, and its molecular mechanisms remain unclear. This study explores the role and regulatory mechanisms of FOSL1 in the wound-healing process. A comprehensive expression dataset of abnormal genes in wound repair was constructed by bioinformatics analysis. Mouse trauma models and mouse wound splint models were constructed to verify the role of FOSL1 in vivo. Real-time quantitative polymerase chain reaction (qRT-PCR), immunoblot, immunofluorescence staining, and HE staining were used to confirm the analysis, and FOSL1 was used as the target in the wound healing process. At the cellular level, using 5'-ethynyl-2'-deoxyuridine (EdU) assay, Transwell assay, Migration assay, western blotting and immunofluorescence, FOSL1 promoted the molecular mechanism of wound repair by regulating the proliferation and migration of keratinocytes through IL-17 signaling pathway. Bioinformatics analysis revealed differential expression of FOSL1 during wound healing. In the mouse back wound model, qRT-PCR, western blotting (WB), and immunofluorescence staining showed significant upregulation of FOSL1 and IL-17 expression during wound tissue healing, indicating a close association between FOSL1 and mouse wound healing. In the mouse wound splinting model, subcutaneous injection of recombinant FOSL1 protein contributed to wound surface healing. Overexpression of FOSL1 in HaCaT cells promoted their proliferation and migration abilities. When IL-17 inhibitor was added to HaCaT cells, both FOSL1 overexpression and knockdown inhibited the proliferation and migration abilities of HaCaT cells. Thus, this study confirms that FOSL1 promotes keratinocyte proliferation and migration through the IL-17 signaling pathway, facilitating wound healing in epidermal wound repair. The results of this study indicate that FOSL1 plays a key role in epidermal wound healing, and elucidate a new molecular mechanism by which FOSL1 promotes keratinocyte proliferation and migration through IL-17 signaling pathway in epidermal wound repair, thereby promoting wound healing.
Keywords: FOSL1; IL-17; Keratinocytes; Wound healing.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval: Yes, the first animal experiment was approved by the Experimental Animal Ethics Committee of Jinan University with the approval number IACUC-20220719-07. The second animal experiment was approved by the Guangzhou Myers Biological Animal Center Experimental Committee with the approval number IACUC-MIS20230074. In conducting the project, we followed the policies of the Nature Portfolio journal and the ARRIVE Guidelines 2.0.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

