Biological markers and psychosocial factors predict chronic pain conditions
- PMID: 40355673
- PMCID: PMC12367528
- DOI: 10.1038/s41562-025-02156-y
Biological markers and psychosocial factors predict chronic pain conditions
Abstract
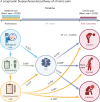
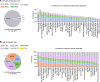
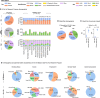
Chronic pain is a multifactorial condition presenting significant diagnostic and prognostic challenges. Biomarkers for the classification and the prediction of chronic pain are therefore critically needed. Here, in this multidataset study of over 523,000 participants, we applied machine learning to multidimensional biological data from the UK Biobank to identify biomarkers for 35 medical conditions associated with pain (for example, rheumatoid arthritis and gout) or self-reported chronic pain (for example, back pain and knee pain). Biomarkers derived from blood immunoassays, brain and bone imaging, and genetics were effective in predicting medical conditions associated with chronic pain (area under the curve (AUC) 0.62-0.87) but not self-reported pain (AUC 0.50-0.62). Notably, all biomarkers worked in synergy with psychosocial factors, accurately predicting both medical conditions (AUC 0.69-0.91) and self-reported pain (AUC 0.71-0.92). These findings underscore the necessity of adopting a holistic approach in the development of biomarkers to enhance their clinical utility.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures











References
-
- Yusuf, E., Kortekaas, M. C., Watt, I., Huizinga, T. W. J. & Kloppenburg, M. Do knee abnormalities visualised on MRI explain knee pain in knee osteoarthritis? A systematic review. Ann. Rheum. Dis.70, 60–67 (2011). - PubMed
-
- Button, K. S. et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci.14, 365–376 (2013). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

