Nicotinamide modulates gut microbial metabolic potential and accelerates recovery in mild-to-moderate COVID-19
- PMID: 40355744
- PMCID: PMC12198012
- DOI: 10.1038/s42255-025-01290-1
Nicotinamide modulates gut microbial metabolic potential and accelerates recovery in mild-to-moderate COVID-19
Abstract
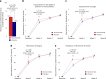
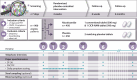
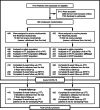
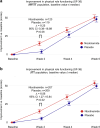
Cellular NAD+ depletion, altered tryptophan metabolism and gut microbiome dysbiosis are associated with disease progression and unfavourable clinical outcomes in COVID-19. Here, we show that supplementing tryptophan metabolism with nicotinamide alleviates COVID-19 symptoms. We evaluate a 4-week intervention with a novel nicotinamide formulation (1,000 mg) in a prospective, double-blind, randomized, placebo-controlled trial in 900 symptomatic outpatients with PCR-proven COVID-19. In the primary analysis population of participants at risk for severe COVID-19, 57.6% of those receiving nicotinamide and 42.6% receiving placebo recover from their performance drop at week 2 (P = 0.004). Nicotinamide is also beneficial for returning to normal activities (P = 0.009). Effects on gut metagenomic signatures parallel clinical efficacy, suggesting that nicotinamide influences COVID-19-associated faecal microbiome changes. After 6 months, responders to nicotinamide in acute COVID-19 show fewer post-COVID symptoms than placebo responders (P = 0.010). No relevant safety signals are observed. Overall, our results show that nicotinamide leads to faster recovery of physical performance and modulates COVID-19-associated faecal microbiome changes.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: S.S. reports indirect stock ownership in Gerion Biotech as well as consulting and personal fees from AbbVie, Allergosan, Amgen, Arena, BMS, Biogen, Celltrion, Celgene, Falk, Ferring, Fresenius, Galapagos/Gilead, HIKMA, I-Mab, Janssen, Lilly, Morphic, MSD, Mylan, Pfizer, Prometheus, Protagonist, Provention Bio, Sandoz/Hexal, Takeda and Theravance. G.H.W. is employed part-time by the CONARIS Research Institute AG (Kiel, Germany). T.B. reports consulting fees, honoraria or other support from AstraZeneca, Boehringer-Ingelheim, Chiesi, GlaxoSmithKline, Merck, MSD, Novartis, Pfizer and Roche. J.M.P. reports stock ownership in Apeiron Biologics and JLP Health. K.A. reports consulting fees, honoraria or other support from AbbVie, Falk, Galapagos, Janssen, Pfizer and Takeda. M.L. reports a lecture honorarium and travel support by AstraZeneca. P.R. reports stock ownership in Gerion Biotech and consulting fees from Takeda. Additional authors of the COVit-2 Study Group: B.B. reports grants, contracts, consulting fees, honoraria or other support from AbbVie, Arena, BMS, Falk, Ferring, Galapagos, Janssen, MSD and Takeda. D.P. reports meeting support from Advanz Pharma. F.T. reports consulting fees, honoraria or other support from AbbVie, Falk, Janssen, L.E.K. Consulting, Lilly and Sanofi. The remaining authors declare no competing interests.
Figures







References
-
- Bogan, K. L. & Brenner, C. Nicotinic acid, nicotinamide, and nicotinamide riboside: a molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu. Rev. Nutr.28, 115–130 (2008). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- EXC 2167: CD-1, CD-2, TI-1/Deutsche Forschungsgemeinschaft (German Research Foundation)
- EXC 2167/Deutsche Forschungsgemeinschaft (German Research Foundation)
- EXC 2167: RTF-VI/Deutsche Forschungsgemeinschaft (German Research Foundation)
- miTARGET (RU5042)/Deutsche Forschungsgemeinschaft (German Research Foundation)
- SO1141/10-1/Deutsche Forschungsgemeinschaft (German Research Foundation)
- SFB1470, SFB1449/Deutsche Forschungsgemeinschaft (German Research Foundation)
- EXC 2167: CD-2, RTF-VI, TI-1/Deutsche Forschungsgemeinschaft (German Research Foundation)
- e:Med Juniorverbund "Try-IBD" 01ZX1915A and 01ZX2215, e:Med Network iTREAT 01ZX2202A/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- e:Med CKDNapp 01ZX1912A/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- K126408/Christian-Albrechts-Universität zu Kiel (Christian-Albrechts-University Kiel)
LinkOut - more resources
Full Text Sources
Medical

