A pragmatic randomized trial to compare strategies for implementing primary HPV testing for routine cervical cancer screening in a large healthcare system
- PMID: 40355876
- PMCID: PMC12067918
- DOI: 10.1186/s13012-025-01432-9
A pragmatic randomized trial to compare strategies for implementing primary HPV testing for routine cervical cancer screening in a large healthcare system
Abstract
Background: Recent updates to national guidelines recommend primary human papillomavirus (HPV) screening for routine cervical cancer screening alongside previously recommended screening options. However, limited guidance exists for implementation approaches that best facilitate cancer screening practice substitution and achieve optimal stakeholder-centered outcomes. We compared "centrally-administered + locally-tailored" (here after referred to as locally-tailored) vs. "centrally-administered + usual care" (here after referred to as centrally-administered) approaches for achieving substitution of HPV and cytology co-testing with primary HPV screening for routine cervical cancer screening to examine the effect of local tailoring on implementation and stakeholder-centered outcomes.
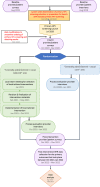
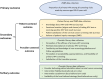
Methods: We conducted a pragmatic, cluster randomized trial embedded in the Kaiser Permanente Southern California (KPSC) health system, randomly assigning site groups to study arms at the level of the geographic service area (12 service area randomized). The study took place between 2020-2022. Centrally-administered implementation strategy bundles included physician and staff educational activities. Sites in the locally-tailored arm underwent local needs assessment followed by local selection, tailoring and deployment of implementation strategy bundles. The primary outcome was the proportion of primary HPV screenings among all screenings performed. Secondary stakeholder-centered outcomes included patient (knowledge, emotional reaction, satisfaction, volume of patient inquiries) and provider outcomes (perception, knowledge, acceptance, and satisfaction) measured via repeated surveys or electronic health records. The generalized estimating equation framework and the difference-in-differences approach were used to compare outcomes across study arms.
Results: The proportion of appropriate screenings (i.e., use of primary HPV screening) during the post-intervention period was high, with no observed difference between study arms: 98.4% (95% confidence interval [CI] 96.3%-100%) for the locally-tailored arm and 99.1% (95% CI: 97.8%-100%) for the centrally-administered arm (p = 0.34). There were no statistically or clinically significant differences in patient- and provider- outcomes between study arms.
Conclusions: Primary HPV screening was feasible and demonstrated high fidelity in all KPSC service areas. The locally-tailored practice substitution approach and centrally-administered practice substitution approach both achieved near complete uptake of primary HPV screening. Further, similar effects on stakeholder-centered outcomes were observed for both approaches. However, generalizability of our findings may be limited due to unique features of our integrated health system.
Trial registration: NCT04371887. Registered 30 April 2020, URL: https://clinicaltrials.gov/study/NCT04371887?cond=primary%20HPV%20screening&rank=5 .
Keywords: Cervical cancer screening; Constrained choice implementation; Embedded research; Implementation approach; Locally-tailored; Practice substitution; Pragmatic trial; Primary HPV screening.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The KPSC IRB reviewed and approved the study. Individuals provided consent to participate in the qualitative interviews. Completion of the study survey was considered implicit consent to participate in the study by the individuals who completed the study surveys. Consent for publication: Not Applicable. Competing interests: None to disclose.
Figures


References
-
- Chatzistamatiou K, Moysiadis T, Moschaki V, Panteleris N, Agorastos T. Comparison of cytology, HPV DNA testing and HPV 16/18 genotyping alone or combined targeting to the more balanced methodology for cervical cancer screening. Gynecol Oncol. 2016;142(1):120–7. 10.1016/j.ygyno.2016.04.027. - DOI - PubMed

