Protein tyrosine phosphatase receptor type kappa (PTPRK) revisited: evolving insights into structure, function, and pathology
- PMID: 40355891
- PMCID: PMC12067748
- DOI: 10.1186/s12967-025-06496-1
Protein tyrosine phosphatase receptor type kappa (PTPRK) revisited: evolving insights into structure, function, and pathology
Abstract
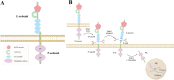
Protein Tyrosine Phosphatase Receptor Type Kappa (PTPRK) is a membrane-bound tyrosine phosphatase encoded by the frequently deleted region of chromosome 6q, which plays a crucial role in regulating cell signaling, adhesion, and immune response. Structurally, PTPRK comprises with an extracellular domain involved in cell-cell adhesion, a transmembrane region, and two intracellular catalytic domains responsible for its phosphatase activity. Notably, PTPRK undergoes proteolytic cleavage by Furin and ADAM10, resulting in the generation of an extracellular E-subunit and a P-subunit. Further processing by γ-secretase releases the intracellular PIC, which plays a pivotal role in regulating β-catenin signaling within the nucleus. PTPRK is widely recognized for its tumor-suppressive properties across various cancers, including colorectal, lung, ovarian, and melanoma. Despite its function as a tumor suppressor, the expression and activity of PTPRK exhibit considerable variability across different cancer types and stages. It exerts its effects by dephosphorylating key signaling molecules such as EGFR, STAT3, CD133 and β-catenin, thereby inhibiting cancer cell proliferation, survival, and metastasis. Beyond its role in cancer, PTPRK is also involved in immune regulation, particularly in the development of CD4 + T cells, and has been implicated in autoimmune diseases such as multiple sclerosis. In the nervous system, PTPRK is linked to neurite outgrowth and synaptic transmission, with genetic polymorphisms in PTPRK associated with an increased risk of neurodegenerative diseases like Alzheimer's disease. Given its extensive involvement in cancer biology, immune regulation, and neurodevelopment, PTPRK presents a promising therapeutic target. Strategies aimed at restoring its activity or targeting PTPRK might offer new approaches for current cancer therapies and overcome drug resistance. In this review, we elucidate the structural characteristics and functional roles of PTPRK in cellular signaling and disease pathogenesis. The variability of PTPRK suggests that the regulatory mechanisms governing its activity are intricate and worth further comprehensive investigation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Consent for publication: None. Abbreviations: Supplementary S1 for details. Competing interests: The authors state that they have no competing interests.
Figures
References
-
- Johnson KG, Van Vactor D. Receptor protein tyrosine phosphatases in nervous system development. Physiol Rev. 2003;83(1):1–24. 10.1152/physrev.00016.2002. - PubMed
-
- Gebbink MF, van Etten I, Hateboer G, et al. Cloning, expression and chromosomal localization of a new putative receptor-like protein tyrosine phosphatase. FEBS Lett. 1991;23(1–2):123–30. 10.1016/0014-5793(91)81241-y. - PubMed
-
- Yang Y, Gil MC, Choi EY, Park SH, Pyun KH, Ha H. Molecular cloning and chromosomal localization of a human gene homologous to the murine R-PTP-kappa, a receptor-type protein tyrosine phosphatase. Gene. 1997;20(1):77–82. 10.1016/s0378-1119(96)00684-1. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous