Treatment of giardiasis in dogs: field clinical study to confirm the efficacy, safety, and acceptance of a metronidazole-based flavored oral suspension
- PMID: 40355903
- PMCID: PMC12067763
- DOI: 10.1186/s13071-025-06797-w
Treatment of giardiasis in dogs: field clinical study to confirm the efficacy, safety, and acceptance of a metronidazole-based flavored oral suspension
Abstract
Background: Giardia duodenalis is a prevalent gastrointestinal parasite in dogs, causing diarrhea, vomiting, and weight loss. Metronidazole is a common treatment for this infection. This study aimed to evaluate the efficacy, safety, and acceptance of a flavored liquid metronidazole oral suspension in treating G. duodenalis in naturally infected dogs.
Methods: A double-masked, vehicle-controlled, randomized, multi-center clinical field trial was conducted. Client-owned dogs with confirmed G. duodenalis infections were enrolled and randomized into AYRADIA-treated and control groups. The AYRADIA group received the metronidazole suspension at 0.2 ml/kg twice daily for 5 days, while the control group received a flavored vehicle suspension without metronidazole. Fecal samples were collected before and after treatment to assess G. duodenalis cyst counts. Clinical examinations and owner assessments were also performed to evaluate safety and treatment acceptance.
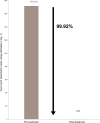
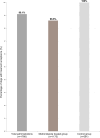
Results: The study enrolled 180 dogs, with 129 included in the efficacy analysis. AYRADIA treatment resulted in a 99.92% reduction in G. duodenalis cyst counts, significantly higher than the reduction in the control group. Adverse events were similar between both groups (10%), mainly consisting of diarrhea and vomiting. The treatment was readily accepted by 99% of dogs.
Conclusions: AYRADIA, administered at 0.2 ml/kg twice daily for 5 days, is highly effective in treating G. duodenalis infections in dogs. The treatment demonstrated a positive safety profile and excellent acceptance. This flavored oral suspension offers a valuable and convenient option for veterinarians managing giardiasis in dogs.
Keywords: Giardia; Canine; Dog; Metronidazole; Treatment.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was approved by Virbac’s animal ethics committee according to Directive 2010/63/EU and the United States authorities’ requirements. Participation in this clinical trial was voluntary. Owners were fully informed about the trial's content and their rights. Signed informed consent was obtained from each dog owner. Consent for publication: Not applicable. Competing interests: All authors were employed by Virbac.
Figures
References
-
- Mateo M, Montoya A, Bailo B, Köster PC, Dashti A, Hernández-Castro C, et al. Prevalence and public health relevance of enteric parasites in domestic dogs and cats in the region of Madrid (Spain) with an emphasis on Giardia duodenalis and Cryptosporidium sp. Vet Med Sci. 2023;9:2542–58. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical