Effect of early adjunctive vasopressin initiation for septic shock patients: a target trial emulation
- PMID: 40355911
- PMCID: PMC12070644
- DOI: 10.1186/s13054-025-05401-y
Effect of early adjunctive vasopressin initiation for septic shock patients: a target trial emulation
Abstract
Background: In septic shock, the optimal timing of adjunctive vasopressin initiation shock is unknown. We aimed to assess the effect of its early initiation for patients with septic shock.
Methods: We conducted a multicenter target trial emulation to estimate the intensive care unit (ICU) mortality effect of early (≤ 6 h) adjunctive vasopressin compared with usual care. Eligible patients had septic shock diagnosed within 6 h of ICU admission. The primary outcome of this study was 30-day ICU mortality. Subgroup analyses were conducted to test the interaction of early vasopressin start with peak norepinephrine-equivalent dose (NED) at 6 h, APACHE score, peak lactate at 6 h and invasive mechanical ventilation. Secondary outcomes were the impact of delayed vasopressin introduction on 30-day ICU mortality and effect of NED at vasopressin start on 30-day ICU mortality. We used the parametric g-formula to emulate a target trial.
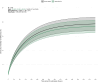
Results: Overall, 3,105 patients fulfilled the inclusion criteria. Mean age was 62 years and mean APACHE III score was 83. In the first six hours of vasopressor therapy, 1,864 (60%) patients were invasively ventilated. Estimated 30-day ICU mortality was 19.34% (95%CI, 17.0 to 21.68) in the no vasopressin group and 18.45% (95%CI, 16.26 to 20.63) in the early vasopressin group; relative risk 0.95 (95%CI, 0.93 to 0.98). The estimated 30-day ICU mortality effect of starting vasopressin was particularly strong at lower norepinephrine doses (< 0.25 µg.kg-1.min-1) and significant at lower norepinephrine doses than recommended by the Surviving Sepsis Campaign Guidelines. Vasopressin administration progressively increased over the study period, from 35.2% (95%CI, 30.0 to 40.5) in 2015 to 45.1% (95%CI, 40.7 to 49.6) in 2021 (ß = + 1.3% per year; 95%CI, + 0.46 to + 2.16, p = 0.011). Patients had progressively lower norepinephrine equivalent dose (ß = - 0.05 µg.kg-1.min-1 per year; 95%CI, - 0.09 to - 0.002, p = 0.038) and lower total SOFA score (ß = - 0.1 point per year; 95%CI, - 0.18 to - 0.07, p < 0.001) at vasopressin start.
Conclusions: In this emulation of a hypothetical target trial, patients with septic shock benefited from early vasopressin administration. These findings can help design prospective randomised-control trials of early adjunctive vasopressin use in septic shock.
Keywords: Critical care; Hypotension; Sepsis; Shock; Vasodilation; Vasopressin.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was approved by the Metro South Hospital and Health Service Human Research Ethics Committee (HREC/2022/QMS/82024) with an individual waiver of consent granted. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures




References
-
- Leone M, Einav S, Antonucci E, Depret F, Lakbar I, Martin-Loeches I, et al. Multimodal strategy to counteract vasodilation in septic shock. Anaesthesia Critic Care Pain Med. 2023;42: 101193. - PubMed
-
- Gordon AC, Mason AJ, Thirunavukkarasu N, Perkins GD, Cecconi M, Cepkova M, et al. Effect of early vasopressin versus norepinephrine on kidney failure in patients with septic shock: the VANISH randomized clinical trial. JAMA. 2016;316:509–18. - PubMed
-
- Sacha GL, Lam SW, Wang L, Duggal A, Reddy AJ, Bauer SR. Association of catecholamine dose, lactate, and shock duration at vasopressin initiation with mortality in patients with septic shock*. Crit Care Med. 2022;50:614–23. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

