Effect of newborn genomic screening for lysosomal storage disorders: a cohort study in China
- PMID: 40355959
- PMCID: PMC12070756
- DOI: 10.1186/s13073-025-01483-z
Effect of newborn genomic screening for lysosomal storage disorders: a cohort study in China
Abstract
Background: Lysosomal storage disorders (LSDs) have a relatively high incidence among rare diseases and can lead to severe consequences if not treated promptly. However, many countries and regions have not included these disorders in their newborn screening programs, resulting in missed early detection, underdiagnosis, and delayed treatment. Newborn genomic screening (NBGS) has shown good screening effectiveness for traditional biochemical screening diseases; however, its effectiveness for LSDs has not yet been evaluated in the general newborn population.
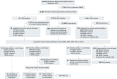
Methods: To evaluate the outcome of NBGS for LSDs, a cohort study was conducted involving newborns recruited from Nanjing Women and Children's Healthcare Hospital in China from March 18, 2022, to September 21, 2023. All participants underwent NBGS of 15 LSDs (18 genes) via dried blood spots, followed by enzyme activity testing for NBGS-positive individuals. The study calculated the incidence and carrier rates for each LSD though NBGS, as well as the positive screening rate, the false positive rate and the positive predictive value of the screening process.
Results: Among 22,687 newborns (11,996 males [52.88%]), 1344 (6.0%) were identified as carriers, and 30 (0.13%) were initially positive for LSDs. Of these, 4 were excluded, 15 were diagnosed as LSD-presymptomatic individuals based on enzyme deficiency and pathogenic variants conforming to inheritance patterns, and 11 remain under follow-up. The estimated combined birth incidence of LSDs in Nanjing was 1/1512, primarily including Fabry disease, Krabbe disease, glycogen storage disease type II, Niemann-Pick disease, and mucopolysaccharidosis type II. Rather than directly comparing NBGS and enzyme activity screening, this study evaluated two sequential screening strategies: (1) NBGS-first with reflex enzyme testing and (2) enzyme activity-first with reflex genomic testing. The NBGS-first strategy demonstrated higher sensitivity and specificity, with a significantly lower false positive rate and higher positive predictive values compared to the enzyme-first strategy (P < 0.05).
Conclusions: This study highlights the potential of NBGS to enhance early detection of presymptomatic LSD individuals, enabling timely interventions and improving newborn health outcomes. Integrating NBGS into routine newborn screening programs could provide an effective and proactive approach for LSD identification and management.
Keywords: Lysosomal storage disorder; Newborn genomic screening; Newborn screening; Rare diseases.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was reviewed and approved by the Ethics Committee of Women's Hospital of Nanjing Medical University (No. 2021KY-071). This research conforms to the principles of the Helsinki Declaration. Written informed consent was obtained from the parents of the participating newborns. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


References
-
- Tandem mass spectrometry in newborn screening. American College of Medical Genetics/American Society of Human Genetics Test and Technology Transfer Committee Working Group. Genet Med. 2000;2(4):267–9. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

