Interactions between time on diet, antibiotic treatment, and liver abscess development on the fecal microbiome of beef cattle
- PMID: 40355979
- PMCID: PMC12067690
- DOI: 10.1186/s42523-025-00413-z
Interactions between time on diet, antibiotic treatment, and liver abscess development on the fecal microbiome of beef cattle
Abstract
Background: Liver abscesses caused by polymicrobial infections of the liver are a widespread problem in feedlot cattle production. There are currently no effective methods for the early detection of liver abscesses or to predict antibiotic efficacy for their control. Although gene expression and microbiome differences have been reported in the rumen of abscessed and normal animals, liver abscess biomarkers using less invasive tools can facilitate managing of the disease in the field.
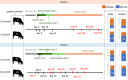
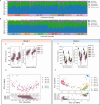
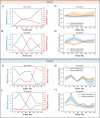
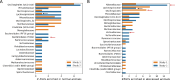
Results: Here we report the results of two studies measuring the fecal microbiome composition of steers that did or did not develop liver abscesses, with or without antibiotic treatment, along a 7-month feeding period on a high-concentrate diet. Our results indicate a limited impact of liver abscesses or tylosin on fecal microbiome composition, with time on diet explaining most variance in the fecal microbiome. Interestingly, in both studies, antibiotic treatment led to larger differences in the variability of the fecal microbiomes between abscessed and normal animals compared to controls. These differences were limited to specific sampling times in each of the two studies. Although multiple amplicon sequence variants with differential abundances according to liver abscess state were identified, there was no overlap between the two studies.
Conclusions: Our results suggest that the fecal abundance of individual microorganisms may not be a robust predictor of liver abscess susceptibility across sampling times or diet regimes. Fecal biomarkers of liver abscess susceptibility might be developed with a focus on other aspects of the hindgut microbiome, especially for animals receiving preventive antibiotics.
Keywords: Antibiotic treatment; Beef cattle; Fecal microbiome; High-energy diets; Liver abscess; Microbial biomarkers.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: All authors were Elanco employees when the study was completed. Elanco sells products containing the antibiotics used as treatments in this study. GP, NTB and DS are employees and own profit interest in BiomEdit, LLC.
Figures
References
LinkOut - more resources
Full Text Sources
