Differences in Dynamics of Specific Antinuclear Antibodies and Their Susceptibility to B Cell-Targeting Treatment in Patients With Systemic Lupus Erythematosus
- PMID: 40356198
- PMCID: PMC12353765
- DOI: 10.1002/art.43219
Differences in Dynamics of Specific Antinuclear Antibodies and Their Susceptibility to B Cell-Targeting Treatment in Patients With Systemic Lupus Erythematosus
Abstract
Objective: The presence of antinuclear antibodies (ANAs) is characteristic for systemic lupus erythematosus (SLE). Antibody dynamics over time are thought to reflect the cellular source of ANAs and their therapeutic targetability. Anti-double-stranded DNA (anti-dsDNA) is the most prevalent and well-studied of all ANAs, and fluctuations in anti-dsDNA serum levels are associated with disease activity. Antibody dynamics of other ANAs, such as antibodies targeting RNA-binding proteins (RBPs), are often considered more stable compared to anti-dsDNA. However, anti-RBPs may be heterogeneous in nature, and their fluctuation has not been extensively analyzed. Therefore, we aimed to study the dynamics of the different ANAs and their susceptibility to B cell-targeting treatments in patients with SLE.
Methods: Seroconversion of specific ANAs was analyzed using electronic health records from patients with SLE. Titers of specific ANAs and anti-varicella zoster virus (VZV) were determined in serum samples from a longitudinal cohort of patients with SLE and serum from patients with SLE treated with rituximab and belimumab.
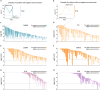
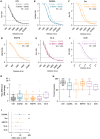
Results: Anti-Smith (anti-Sm) and anti-RNP, similar to anti-dsDNA titers, seroconverted more frequently compared to anti-SS-A/SS-B. Furthermore, anti-Sm/RNP and anti-dsDNA titers, but not anti-SS-A/SS-B titers, fluctuated significantly compared to stable anti-VZV controls. Likewise, reductions in anti-dsDNA and anti-Sm/RNP titers after B cell-depleting treatment were comparable in magnitude. However, only anti-dsDNA titer reductions associated with clinical outcomes.
Conclusion: Together, these results show that anti-Sm/RNP and anti-dsDNA, in contrast to anti-SS-A/SS-B, frequently fluctuate and their levels can decrease following B cell-targeted treatments. Thus, distinct ANAs have variable kinetics, potentially reflecting their derivation from different B cell differentiation pathways.
© 2025 The Author(s). Arthritis & Rheumatology published by Wiley Periodicals LLC on behalf of American College of Rheumatology.
Figures



References
-
- Bombardier C, Gladman DD, Urowitz MB, et al; The Committee on Prognosis Studies in SLE. A disease activity index for lupus patients. Arthritis Rheum 1992;35(6):630–640. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

