Innate Lymphoid Cell Phenotypic and Functional Alterations in Patients With Systemic Juvenile Idiopathic Arthritis
- PMID: 40356210
- PMCID: PMC12479180
- DOI: 10.1002/art.43217
Innate Lymphoid Cell Phenotypic and Functional Alterations in Patients With Systemic Juvenile Idiopathic Arthritis
Abstract
Objective: Systemic juvenile idiopathic arthritis (sJIA) is a chronic childhood disease classically attributed to innate immune cell dysregulation. This study aimed to elucidate the role of innate lymphoid cells (ILCs), including natural killer (NK) cells and helper-ILCs (hILCs), in sJIA during clinically inactive disease (CID) through phenotypic and functional analysis.
Methods: Peripheral ILCs from children with sJIA during CID receiving interleukin-1 (IL-1) inhibitors (n = 40) were analyzed by flow cytometry and compared to 23 healthy children (HC) and 22 patients with unrelated autoinflammatory diseases taking IL-1 inhibitors. Plasma proteomic profiling was also performed.
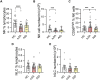
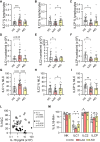
Results: Patients with sJIA showed a significant reduction in circulating NK cell frequencies compared to HC, with an increased proportion of CD56bright NK cells. Although overall hILC frequencies were comparable to HC, ILC1s were increased, whereas ILC precursors were reduced. ILC1 frequency correlated positively with IL-18 plasma levels, whereas ILC2 frequency correlated negatively. Functional assessments revealed that NK cells from patients with sJIA had variable interferon γ (IFNγ) production upon IL-18/IL-12 stimulation, inversely correlating with IL-18 levels. Additionally, hILCs from these patients showed a specific impairment in IFNγ production despite normal IL-13 production, potentially linked to decreased IL-18 receptor α expression in ILC1s. Proteomic analysis confirmed IL-18 as the most up-regulated cytokine in sJIA plasma.
Conclusion: Patients with sJIA in CID exhibit significant innate immune abnormalities, including altered ILC subset distribution and impaired IFNγ production, strongly associated with IL-18 levels. These findings suggest ongoing immune dysregulation despite clinical remission, underscoring a potential role for ILCs and cytokine interaction in sJIA pathogenesis.
© 2025 The Author(s). Arthritis & Rheumatology published by Wiley Periodicals LLC on behalf of American College of Rheumatology.
Figures

References
-
- Vivier E, Artis D, Colonna M, et al. Innate lymphoid cells: 10 years on. Cell 2018;174(5):1054–1066. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

