A Systematic Review of Immune Cell Roles in Breast Cancer Immunotherapy
- PMID: 40356222
- PMCID: PMC12069222
- DOI: 10.1002/cnr2.70217
A Systematic Review of Immune Cell Roles in Breast Cancer Immunotherapy
Abstract
Background: Breast cancer (BC) is the most prevalent malignancy among women and is associated with high mortality and significant clinical challenges. Although conventional treatments such as surgery, chemotherapy, and radiotherapy have significantly improved patient survival, their efficacy remains limited by severe side effects and treatment resistance. In recent years, advances in immunotherapy have underscored the pivotal role of immune cells in treating BC.
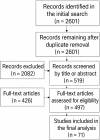
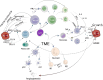
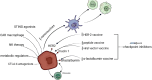
Recent findings: This systematic review summarizes the current knowledge on the roles of immune cells within the BC tumor microenvironment (TME), including their phenotypes, functions, and implications for immunotherapy. Following PRISMA guidelines, 71 studies published between 2010 and 2024 were analyzed. The results indicate that immune cell populations-such as tumor-associated macrophages (TAMs), tumor-infiltrating lymphocytes (TILs), natural killer (NK) cells, dendritic cells (DCs), and myeloid-derived suppressor cells (MDSCs)-are integral to tumor progression and therapeutic response. However, their functional heterogeneity and plasticity remain key obstacles to the development of effective and personalized immunotherapeutic strategies.
Conclusion: Further research is needed to clarify the mechanisms governing immune cell behavior within the BC TME and to advance precision immunotherapy. Such insights will lay the foundation for individualized treatment approaches, ultimately improving patient outcomes and quality of life (QoL).
Keywords: breast cancer; immune cells; immunotherapy; tumor microenvironment; vaccines.
© 2025 The Author(s). Cancer Reports published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Guardians and Mediators of Metastasis: Exploring T Lymphocytes, Myeloid-Derived Suppressor Cells, and Tumor-Associated Macrophages in the Breast Cancer Microenvironment.Int J Mol Sci. 2024 Jun 5;25(11):6224. doi: 10.3390/ijms25116224. Int J Mol Sci. 2024. PMID: 38892411 Free PMC article. Review.
-
Tumor microenvironment of human breast cancer, and feline mammary carcinoma as a potential study model.Biochim Biophys Acta Rev Cancer. 2021 Aug;1876(1):188587. doi: 10.1016/j.bbcan.2021.188587. Epub 2021 Jul 5. Biochim Biophys Acta Rev Cancer. 2021. PMID: 34237352 Review.
-
Modulation of tumor microenvironment for immunotherapy: focus on nanomaterial-based strategies.Theranostics. 2020 Feb 10;10(7):3099-3117. doi: 10.7150/thno.42998. eCollection 2020. Theranostics. 2020. PMID: 32194857 Free PMC article. Review.
-
Role of Tumor-Associated Macrophages in Breast Cancer Immunotherapy.Front Biosci (Landmark Ed). 2025 Apr 23;30(4):26995. doi: 10.31083/FBL26995. Front Biosci (Landmark Ed). 2025. PMID: 40302326 Review.
-
Role of immune regulatory cells in breast cancer: Foe or friend?Int Immunopharmacol. 2021 Jul;96:107627. doi: 10.1016/j.intimp.2021.107627. Epub 2021 Apr 14. Int Immunopharmacol. 2021. PMID: 33862552 Review.
References
-
- Pei J., Peng Y., Ma K., et al., “Integrated Analysis Reveals FLI1 Regulates the Tumor Immune Microenvironment via Its Cell‐Type‐Specific Expression and Transcriptional Regulation of Distinct Target Genes of Immune Cells in Breast Cancer,” BMC Genomics 25, no. 1 (2024): 250, 10.1186/s12864-024-10174-9. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

