Dissolution Characteristics of Split and Crushed Levetiracetam Extended-Release Tablets in Comparison With Immediate-Release Formulation
- PMID: 40356306
- PMCID: PMC12257259
- DOI: 10.1111/jvp.13517
Dissolution Characteristics of Split and Crushed Levetiracetam Extended-Release Tablets in Comparison With Immediate-Release Formulation
Abstract
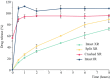
Levetiracetam (LEV) is a commonly used antiseizure medication in dogs, available in immediate-release (LEV-IR) and extended-release (LEV-XR) formulations. LEV-XR improves owner compliance with less frequent dosing, but its lowest concentration tablet (500 mg) often exceeds recommended doses for small dogs. This study evaluated how modifying LEV-XR tablets affects dissolution rates, comparing intact, split, and crushed LEV-XR tablets with intact LEV-IR tablets. Dissolution testing followed United States Pharmacopeia (USP) guidelines for LEV-XR tablets. Tablets were placed in a buffer solution (pH 6.0) and agitated at 100 rpm. Samples were collected at 0, 0.5, 2, 4, 6, and 8 h, then analyzed by high-pressure liquid chromatography (HPLC) using a USP reference standard. Results indicated that splitting LEV-XR tablets slightly increased drug release compared to intact tablets, while crushing eliminated extended-release properties, mimicking LEV-IR dissolution. These findings suggest that splitting LEV-XR tablets may be a viable strategy for dosing small dogs without compromising sustained release. Conversely, crushing LEV-XR tablets may be useful for rapid drug release in cluster seizure protocols. Future pharmacokinetic studies are needed to confirm if these in vitro results correlate with in vivo performance for both maintenance and emergency seizure management in dogs.
Keywords: antiseizure medication; canine epilepsy; cluster seizure protocol; in vitro dissolution; tablet modification.
© 2025 The Author(s). Journal of Veterinary Pharmacology and Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Booth, S. , Meller S., Packer R. M. A., Farquhar R., Maddison J. E., and Volk H. A.. 2021. “Owner Compliance in Canine Epilepsy.” Veterinary Record 188, no. 4: 262–269. - PubMed
-
- Lynch, B. A. , Lambeng N., Nocka K., et al. 2004. “The Synaptic Vesicle Protein SV2A is the Binding Site for the Antiepileptic Drug Levetiracetam.” Proceedings of the National Academy of Sciences of the United States of America 101, no. 26: 9861–9866. www.pnas.orgcgidoi10.1073pnas.0308208101. - PMC - PubMed
-
- Lyseng‐Williamson, K. 2011. “Levetiracetam A Review of Its Use in Epilepsy.” Drugs 71, no. 4: 489–514. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources