Untargeted metabolic analysis in serum samples reveals metabolic signature in children with congenital heart failure on enalapril therapy
- PMID: 40356784
- PMCID: PMC12066549
- DOI: 10.3389/fped.2025.1530063
Untargeted metabolic analysis in serum samples reveals metabolic signature in children with congenital heart failure on enalapril therapy
Abstract
Introduction: Enalapril is an angiotensin-converting enzyme (ACE) inhibitor (ACEi) which is widely used in the management of (paediatric) hypertension and heart failure (HF). There is a significant interindividual variability in the patient's response to enalapril that is not completely understood. Therefore, we aimed to examine the potential of metabolic profiling for stratifying paediatric patients with HF due to congenital heart disease (CHD) in terms of treatment response to enalapril. Additionally, we investigated metabolic profiles in CHD patients and healthy controls.
Methods: CHD patients aged 0-6 years of age who previously participated in a multi-centre and multinational pharmacokinetic safety bridging study of enalapril were included. Patients were defined as responder when aldosterone levels decreased after a single administration of enalapril. Non-responders were those with an increase in their aldosterone levels. We applied an untargeted mass spectrometry-based metabolomics approach on serum. By using both supervised and unsupervised learning algorithms, we compared metabolic profiles between responders and non-responders as well as between patients and age and sex matched healthy controls.
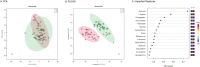
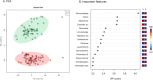
Results: In total, 63 patients were included with a median age of 132 (IQR 54-211) days and 46 controls [97 (63-160) days]. 41 of 63 patients responded to enalapril therapy. Their baseline characteristics were similar to non-responders (n = 22). A total of 1,820 unique features were identified. Responders were distinguished from non-responders using a supervised learning algorithm based on 94 features (p = 0.05). Furthermore, metabolic profiles could distinguish between patients and controls based on an unsupervised learning algorithm which revealed 278 relevant features (p = 0.001).
Conclusions: These are the first data to demonstrate a clear metabolic signature in children with CHD using ACEi. We identified metabolites whose concentrations were both associated with ACEi response and HF. This indicates more severe HF in patients with more profound treatment response. Our results will therefore allow further studies aiming at disentangling variability in ACEi treatment response.
Keywords: ACE inhibitor; children; enalapril; heart failure; pharmacokinetics.
© 2025 Smeets, van Hoek, Jans, Dalinghaus, Laer, Bajcetic, Male and de Wildt.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Konstam MA, Kronenberg MW, Rousseau MF, Udelson JE, Melin J, Stewart D, et al. Effects of the angiotensin converting enzyme inhibitor enalapril on the long-term progression of left ventricular dilatation in patients with asymptomatic systolic dysfunction. SOLVD (studies of left ventricular dysfunction) investigators. Circulation. (1993) 88(5 Pt 1):2277–83. 10.1161/01.CIR.88.5.2277 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

