Mimicking immune complexes for efficient antibody responses
- PMID: 40356891
- PMCID: PMC12066251
- DOI: 10.3389/fimmu.2025.1570487
Mimicking immune complexes for efficient antibody responses
Abstract
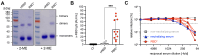
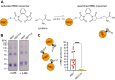
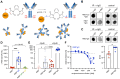
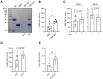
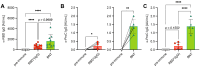
Efficient antibody responses are crucial for combating infectious diseases and vaccination remains a cornerstone of this effort. This study introduces a novel approach for enhancing immune responses in wild-type mice by utilizing pre-formed immune complexes, using the receptor-binding domain (RBD) of SARS-CoV-2 as a model antigen to illustrate the broader potential of the concept. Specifically, we found that pre-treating the antigen with bis-maleimide, a chemical linker that facilitates protein cross-linking, significantly enhances antibody production. Moreover, in vitro cross-linking of antigen to unrelated IgG using bis-maleimide generated immune complexes that markedly enhanced antigen-specific antibody responses, likely by mimicking natural memory-like mechanisms, suggesting that bis-maleimide pre-treated antigens may similarly engage IgG in vivo. In contrast, antigen crosslinking with IgA or IgM did not yield comparable effects, highlighting the unique capacity of IgG to boost immunogenicity. By leveraging the principles of immune memory, this study demonstrates the potential of pre-formed immune complexes to significantly enhance vaccine efficacy using an antigen-independent strategy broadly applicable to diverse pathogens.
Keywords: B cells; IgG; adjuvant; antibody responses; memory.
Copyright © 2025 Schönfelder, El Ayoubi, Havryliuk, Groß, Seidel, Bakchoul, Münch, Jumaa and Setz.
Conflict of interest statement
HJ and CS have filed an invention disclosure for “activated antigens” and authorized Vaccinvent GmbH and Ulm University to obtain title of the patent. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

