Incidence of infection associated with eculizumab: a meta-analysis of 9 randomized controlled trials
- PMID: 40356951
- PMCID: PMC12067414
- DOI: 10.3389/fphar.2025.1538563
Incidence of infection associated with eculizumab: a meta-analysis of 9 randomized controlled trials
Abstract
Background and aims: Eculizumab is expected to lead to increased susceptibility to infection. We performed a meta-analysis of data from randomized controlled trials (RCTs) to determine the risk of infection in eculizumab-treated patients.
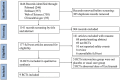
Methods: We searched PubMed, EMBASE, Web of Science and ClinicalTrials.gov (up to 8 Oct 2024) to identify published RCTs that focused on the occurrence of infection in patients treated with eculizumab regardless of the indications of the patients. Relative risks and 95% confidence intervals (95% CIs) were calculated via the random effects model. (PROSPERO Code No. CRD42024562470).
Results: Nine RCTs including 691 patients were eligible. Compared with the control (placebo or standard of care), eculizumab did not significantly increase the overall risk of infection (RR = 1.07; 95% CI, 0.89-1.28; I 2 = 44%), regardless of whether the infection was a general infection (RR = 1.07; 95% CI, 0.86-1.34; I 2 = 39%) or a serious infection (RR = 1.05; 95% CI, 0.75-1.47; I 2 = 11%). Analyses of subgroups revealed that eculizumab significantly increased the risk of general urinary system infection (RR = 1.33; 95% CI, 1.00-1.77; I 2 = 46%) and severe bacteremia (RR = 2.31; 95% CI, 1.04-5.13; I 2 = 0%).
Conclusion: Compared with placebo or standard of care, although eculizumab did not significantly increase the overall risk of infection, it was associated with 33% and 131% increases in the risk of general urinary system infection and severe bacteremia, respectively.
Systematic review registration: PROSPERO CRD42024562470.
Keywords: bacteremia; eculizumab; infection; meta-analysis; urinary tract infection.
Copyright © 2025 Jiang, Liu, Wei and Xiao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Incidence and Risk of Infection Associated With Fingolimod in Patients With Multiple Sclerosis: A Systematic Review and Meta-Analysis of 8,448 Patients From 12 Randomized Controlled Trials.Front Immunol. 2021 Mar 8;12:611711. doi: 10.3389/fimmu.2021.611711. eCollection 2021. Front Immunol. 2021. PMID: 33763062 Free PMC article.
-
Interleukin-6 blocking agents for treating COVID-19: a living systematic review.Cochrane Database Syst Rev. 2021 Mar 18;3(3):CD013881. doi: 10.1002/14651858.CD013881. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2023 Jun 1;6:CD013881. doi: 10.1002/14651858.CD013881.pub2. PMID: 33734435 Free PMC article. Updated.
-
Pharmacological treatment other than corticosteroids, intravenous immunoglobulin and plasma exchange for Guillain-Barré syndrome.Cochrane Database Syst Rev. 2020 Jan 25;1(1):CD008630. doi: 10.1002/14651858.CD008630.pub5. Cochrane Database Syst Rev. 2020. PMID: 31981368 Free PMC article.
-
Use of GLP-1 Receptor Agonists and Occurrence of Thyroid Disorders: a Meta-Analysis of Randomized Controlled Trials.Front Endocrinol (Lausanne). 2022 Jul 11;13:927859. doi: 10.3389/fendo.2022.927859. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35898463 Free PMC article.
-
Remdesivir for the treatment of COVID-19.Cochrane Database Syst Rev. 2021 Aug 5;8(8):CD014962. doi: 10.1002/14651858.CD014962. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2023 Jan 25;1:CD014962. doi: 10.1002/14651858.CD014962.pub2. PMID: 34350582 Free PMC article. Updated.
References
-
- Gäckler A., Schönermarck U., Dobronravov V., La Manna G., Denker A., Liu P., et al. (2021). Efficacy and safety of the long-acting C5 inhibitor ravulizumab in patients with atypical hemolytic uremic syndrome triggered by pregnancy: a subgroup analysis. BMC Nephrol. 22, 5. 10.1186/s12882-020-02190-0 - DOI - PMC - PubMed
-
- Garnier A., Brochard K., Kwon T., Sellier-Leclerc A.-L., Lahoche A., Launay E. A., et al. (2023). Efficacy and safety of eculizumab in pediatric patients affected by shiga toxin-related hemolytic and uremic syndrome: a randomized, placebo-controlled trial. A Randomized, Placebo-Controlled Trial. JASN 34, 1561–1573. 10.1681/ASN.0000000000000182 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources