Investigating the regulation of the miR-199a-3p/TGF-β/Smad signaling pathway by BSHXF drug-containing serum combined with ADSCs for delaying intervertebral disc degeneration
- PMID: 40356987
- PMCID: PMC12067415
- DOI: 10.3389/fphar.2025.1583635
Investigating the regulation of the miR-199a-3p/TGF-β/Smad signaling pathway by BSHXF drug-containing serum combined with ADSCs for delaying intervertebral disc degeneration
Abstract
Background: Intervertebral disc degeneration (IDD) significantly contributes to low back pain (LBP), yet effective treatment options are scarce. BSHXF, a classical traditional Chinese medicine formula, demonstrates dual pharmacological actions: tonifying kidneys, strengthening bones, activating blood circulation, and resolving stasis. It has been widely used in IDD management. Given its potential, combining BSHXF with miRNA regulation and stem cell therapy may enhance therapeutic outcomes by targeting molecular and cellular pathways underlying IDD pathogenesis.
Aim of the study: IDD is recognized as one of the primary causes of low back pain, yet effective therapeutic interventions for this condition remain limited. This study explores the role of BSHXF drug-containing serum combined with adipose-derived stem cells (ADSCs) in slowing IDD progression via the miR-199a-3p/TGF-β/Smad signaling pathway. By comprehensively investigating the synergistic effects of this combination therapy, we aim to propose a novel multi-target strategy that addresses the complex pathogenesis of IDD.
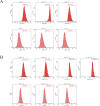
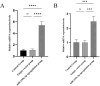
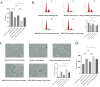
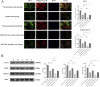
Materials and methods: This study employed a combination of in vivo and in vitro models. An IDD model was induced in rat caudal intervertebral discs through needle puncture, while an oxidative stress-induced ADSCs injury model was created in vitro using tert-butyl hydroperoxide (T-BHP). Cell viability was measured with the CCK-8 assay. Cell cycle distribution and mitochondrial reactive oxygen species (ROS) levels were assessed using flow cytometry. Cellular senescence was assessed using SA-β-galactosidase staining. Lactate dehydrogenase (LDH) activity was quantified to evaluate cellular damage. Differentiation into nucleus pulposus-like cells was assessed using immunofluorescence double staining for CD73 and COL2A1. ELISA was used to measure inflammatory cytokines (TNF-α, IL-1β, IL-4, IL-10) in cell supernatants. miR-199a-3p expression was determined using RT-qPCR. Western blotting was employed to quantify COL2A1, SOX9, and ACAN protein levels, reflecting nucleus pulposus-like differentiation and extracellular matrix (ECM) synthesis capacity. Western blotting was employed to assess pathway activity by analyzing the protein expressions of TGF-β1, Smad2, Smad3, and their phosphorylated forms, P-Smad2 and P-Smad3. In vivo experiments assessed histopathological degeneration through hematoxylin-eosin (HE) and Safranin O-Fast Green staining. Immunohistochemistry (IHC) analyzed COL1A1 and COL2A1 expression levels. RT-qPCR quantified miR-199a-3p expression. Western blotting was employed to assess the expression levels of TGF-β1, Smad2, Smad3, P-Smad2, and P-Smad3 for pathway regulation evaluation.
Results: Our experimental results demonstrated that serum containing BSHXF significantly alleviated T-BHP-induced oxidative stress, improved the cellular microenvironment, promoted ADSCs proliferation, and decelerated cellular senescence. Further mechanistic analysis revealed that BSHXF significantly activated the TGF-β/Smad signaling pathway, driving the differentiation of ADSCs into nucleus pulposus-like cells and restoring normal cell cycle progression. Overexpression of miR-199a-3p inhibited the TGF-β/Smad pathway, leading to ECM degradation and elevated expression of inflammatory factors (TNF-α, IL-1β). In contrast, BSHXF restored TGF-β/Smad pathway activity by downregulating miR-199a-3p expression. In vivo experiments demonstrated that miR-199a-3p overexpression exacerbated IDD, characterized by reduced COL2A1 expression, elevated COL1A1 levels, and increased disc fibrosis. BSHXF intervention markedly attenuated IDD progression by downregulating miR-199a-3p expression, reducing disc fibrosis, and effectively restoring collagen expression.
Conclusion: BSHXF activated the TGF-β/Smad pathway to promote the differentiation of ADSCs into nucleus pulposus-like cells. It exerted protective effects by alleviating oxidative stress damage, improving the microenvironment, delaying senescence, and enhancing cellular functions. This study is the first to reveal that miR-199a-3p overexpression exacerbates intervertebral disc fibrosis and degeneration. BSHXF restored TGF-β/Smad pathway activity by downregulating miR-199a-3p expression, thereby improving disc structure and function. This integrated approach offers a novel multi-target intervention strategy for IDD, demonstrating significant therapeutic potential.
Keywords: adipose-derived stem cells; extracellular matrix synthesis; intervertebral disc degeneration; miR-199a-3p/TGF-β/Smad signaling pathway; oxidative stress.
Copyright © 2025 Liu, Sun, Yang, Jiang, Sun, Chen, Duan and Yang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

