Tremors and Health-Related Quality of Life in Liver Transplant Recipients: Post-hoc Analysis of a Multicenter, Randomized, Controlled Trial Comparing a Life Cycle Pharma-Tacrolimus Regimen and Extended-Release Tacrolimus Regimen
- PMID: 40357166
- PMCID: PMC12066294
- DOI: 10.3389/ti.2025.14189
Tremors and Health-Related Quality of Life in Liver Transplant Recipients: Post-hoc Analysis of a Multicenter, Randomized, Controlled Trial Comparing a Life Cycle Pharma-Tacrolimus Regimen and Extended-Release Tacrolimus Regimen
Abstract
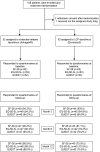
We investigated whether life cycle pharma (LCP)-tacrolimus compared to extended-release (ER)-tacrolimus results in a difference in severity of tremors and HRQoL. In this multi-center, open-label, randomized, controlled trial, 108 patients were randomized in a 1:1 ratio to either LCP-tacrolimus regimen or ER-tacrolimus regimen after transplantation. HRQoL was assessed with the EQ-5D-5L and SF-36 questionnaire (two generic HRQoL instruments) and the quality of life in essential tremor (QUEST) questionnaire (domain specific HRQoL instrument). The EQ-5D-5L scores were translated to the societal values. We examined the HRQoL over the course of the study by fitting generalized mixed effect models. In total, 105 patients were included, 53 to the LCP- and 52 to the ER-tacrolimus regimen. Baseline questionnaires were available for every LT recipient. At 12 months 25% [10/40], 95% confidence interval (CI) 14.2%-40.2% of the LT recipients in the LCP-tacrolimus regimen group experienced tremors compared to 30.4% [14/46], 95%-CI 19.1%-44.8% of the LT recipients in the ER-tacrolimus regimen group; risk difference: 0.054; 95%-CI -0.151-0.249; p = 0.63. No statistically significant differences in HRQoL were seen between the two regimens. We could not demonstrate differences in the HRQoL or occurrence of tremors between LCP-tacrolimus and ER-tacrolimus regimens.
Keywords: healthrelated quality of life; immunosuppressive therapy; liver transplantation; tacrolimus; tremors.
Copyright © 2025 Mulder, Busschbach, van Hoek, Polak, Alwayn, de Winter, Murad, Verhey-Hart, Elshove, Erler, Hesselink, den Hoed and Metselaar.
Conflict of interest statement
MM has received lecture fees and consulting fees from Chiesi Pharma and grant support from Astellas Pharma. MM does not have employment or stock ownership at any of these companies, and neither does he have patents or patent applications. DH has received lecture fees and consulting fees from Astellas Pharma, Astra Zeneca, Chiesi Pharma, Medincell, Novartis Pharma, Sangamo Therapeutics and Vifor Pharma. He has received grant support from Astellas Pharma, Bristol-Myers Squibb and Chiesi Pharma (paid to his institution). DH does not have employment or stock ownership at any of these companies, and neither does he have patents or patent applications. CH has received lecture fees and consulting fees from Chiesi Pharma, Takeda, Novartis Pharma, Abacus medical and travel grants from Orphalan. CH does not have employment or stock ownership at any of these companies, and neither does she have patents or patent applications. HM has received lecture fees from Astellas Pharma and received grant support from Astellas Pharma, Novartis Pharma and Chiesi Pharma. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Mulder MB, Busschbach JV, van Hoek B, van den Berg AP, Polak WG, Alwayn IPJ, et al. Health-related Quality of Life and Fatigue in Liver Transplant Recipients Receiving Tacrolimus versus Sirolimus-Based Immunosuppression: Results from a Randomized Trial. Transplantation (2023) 107:2545–53. 10.1097/TP.0000000000004619 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

