Cost-effectiveness analysis of second-line medical therapies in acromegaly: a real-life study
- PMID: 40357207
- PMCID: PMC12066263
- DOI: 10.3389/fendo.2025.1573721
Cost-effectiveness analysis of second-line medical therapies in acromegaly: a real-life study
Abstract
Introduction: Acromegaly is an uncommon disease with important comorbidity and economic cost. Although the pharmacological cost of second-line treatment for refractory acromegaly has been theoretically analyzed, real-life studies are needed.
Objectives: To assess the use of pasireotide and pegvisomant in a third-level center under routine clinical practice.
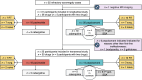
Methods: Acromegaly patients that had been treated with pasireotide and/or pegvisomant were included in (A) a cross-sectional study (two years after starting these drugs) to analyze the cost of acromegaly, hormone replacement, and type 2 diabetes mellitus (T2DM) treatments, and the cost of surgery and radiotherapy; and (B) a retrospective cohorts study (May 2006-October 2024) to analyze efficacy, safety (adverse events, fasting glucose, glycated hemoglobin, and T2DM diagnosis), and dose evolution. Descriptive statistics were 10% trimmed means and standard deviation. Two-tailed hypothesis testing with Yuen's t and Fisher's test had a P < 0.05 significance.
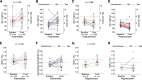
Results: 25 participants were included in the transversal study and 31 participants in the longitudinal study. A typical patient with a poorly granulated GH-producing adenoma underwent in-center surgery once and received radiotherapy. In the transversal study, total pharmacological cost was 34,139.29 (13,472.09) €/person/year, with 33,874.88 (13,468.36) €/person/year for second-line acromegaly drugs. Pasireotide displayed 9,423.26 €/person/year worth of savings (P = .12), reaching 30,415.98 €/person/year at high dose (P < 0.001). In the longitudinal study, pasireotide dose was reduced (P = .06) regardless of treatment modality. Pasireotide affected carbohydrate metabolism (P = .001), but the effect was generally mild.
Conclusions: Pasireotide was found to be a more cost-effective option in patients with first-line treatment failure.
Keywords: acromegaly; cost analysis; pasireotide; pegvisomant; radiotherapy.
Copyright © 2025 Venegas Moreno, Jiménez-Sánchez, Remón-Ruiz, Dios, Perea Cortés, Hernández-Reina, Cano and Soto Moreno.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships acting as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

