SiO2@Benzothiazole-Cl@Fc as an Efficient Heterogeneous Catalyst for the Synthesis of 1,3,5-Trisubstituted Pyrazoles by A3 Coupling
- PMID: 40357693
- PMCID: PMC12368891
- DOI: 10.1002/open.202500024
SiO2@Benzothiazole-Cl@Fc as an Efficient Heterogeneous Catalyst for the Synthesis of 1,3,5-Trisubstituted Pyrazoles by A3 Coupling
Abstract
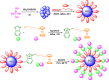
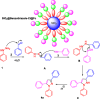
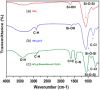
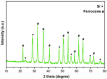
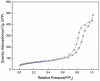
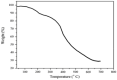
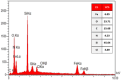
This research introduces the preparation and analysis of a newly heterogeneous catalyst developed silica nanospheres supporting a ferrocene-containing ionic liquid (IL) (SiO2@Benzothiazole-Cl@Fc) for the A3 coupling reaction. The catalyst facilitates the efficient synthesis of 1,3,5-trisubstituted pyrazoles from aromatic hydrazides, aldehydes, and aromatic alkynes. Incorporating ferrocene enhances the catalytic activity. Comprehensive characterization techniques, including NMR, Fourier transform infrared, transmission electron microscopy, energy-dispersive X-ray spectroscopy, and scanning electron microscopy, confirm the successful functionalization of silica nanospheres. The catalytic performance was evaluated under various reaction conditions, demonstrating high yields and selectivity for the desired pyrazole products. This work highlights the potential of ferrocene-based ILs in green chemistry applications, providing a sustainable approach to synthesizing valuable heterocyclic compounds.
Keywords: A3 coupling; ferrocene; heterogeneous catalyst; ionic liquid; pyrazole synthesis; silica nanospheres.
© 2025 The Author(s). ChemistryOpen published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Zhu J., Wang Q., Wang M. X. (Eds.), Multicomponent Reactions in Organic Synthesis, Wiley‐VCH, Weinheim: 2015, 1.
-
- Multicomponent Reactions 1: Reactions Involving a Carbonyl Compound as Electrophilic Component, Science of Synthesis: Multicomponent Reactions 2014.
LinkOut - more resources
Full Text Sources

