Lipid-Lowering Effect and Safety of Ezetimibe and Atorvastatin 5 mg in Patients With Primary Hypercholesterolemia or Mixed Dyslipidemia: A Randomized, Double-Blind, Parallel, Multicenter, Phase 3 Clinical Trial
- PMID: 40357888
- PMCID: PMC12070249
- DOI: 10.1002/clc.70138
Lipid-Lowering Effect and Safety of Ezetimibe and Atorvastatin 5 mg in Patients With Primary Hypercholesterolemia or Mixed Dyslipidemia: A Randomized, Double-Blind, Parallel, Multicenter, Phase 3 Clinical Trial
Abstract
Objective: This study aimed to compare the lipid-lowering effect and safety of low-intensity atorvastatin (5 mg) plus ezetimibe (10 mg) combination therapy (A5E10) with monotherapy regimens-atorvastatin 5 mg [A5], ezetimibe 10 mg [E10], and atorvastatin 10 mg [A10])-in dyslipidemia patients.
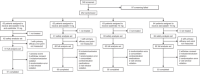
Methods: A randomized, double-blind, placebo-controlled trial involving 252 dyslipidemia patients was conducted at 25 centers in South Korea (NCT05970679). Participants aged ≥ 19 years were randomized into four groups: A5E10, A5, E10, and A10. The primary endpoint was the percentage change in low-density lipoprotein cholesterol (LDL-C) levels from baseline to 8 weeks. Secondary endpoints included changes in other lipid parameters, lipid ratios, LDL-C goal achievement rates and safety assessments.
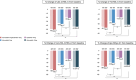
Results: The mean age of the patients was 63 years, and 51.2% were male. The A5E10 group showed significantly greater LDL-C reduction (47.6%) compared with A5 (33.4%), E10 (19.4%), and A10 (40.1%) at 8 weeks (p < 0.0001). A5E10 also significantly reduced triglyceride, non-high-density lipoprotein cholesterol, and apolipoprotein B levels. In addition, a significant reduction in LDL-C levels was observed over the 4 weeks, with a 46.7% reduction in LDL-C levels after 4 weeks of A5E10 administration. No severe adverse events were observed in the A5E10 group.
Conclusion: The combination of low-intensity atorvastatin and ezetimibe was more effective than moderate-intensity atorvastatin monotherapy in lowering LDL-C levels and improving other lipid parameters. It was well-tolerated and demonstrated rapid benefits within a month, offering a promising alternative for patients with low to moderate cardiovascular risk who do not achieve adequate control with statin monotherapy.
Keywords: atorvastatin; cholesterol; ezetimibe; statin.
© 2025 The Author(s). Clinical Cardiology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Baigent C., Keech A., Kearney P. M., et al., “Efficacy and Safety of Cholesterol‐Lowering Treatment: Prospective Meta‐Analysis of Data From 90,056 Participants in 14 Randomised Trials of Statins,” Lancet (London, England) 366, no. 9630 (2005): 1267–1278, 10.1016/S0140-6736(05)67394-1. - DOI - PubMed
-
- Long‐Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group , “Prevention of Cardiovascular Events and Death With Pravastatin in Patients With Coronary Heart Disease and a Broad Range of Initial Cholesterol Levels,” New England Journal of Medicine 339, no. 19 (1998): 1349–1357, 10.1056/NEJM199811053391902. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

