Detection of S1 spike protein in CD16+ monocytes up to 245 days in SARS-CoV-2-negative post-COVID-19 vaccine syndrome (PCVS) individuals
- PMID: 40358138
- PMCID: PMC12077440
- DOI: 10.1080/21645515.2025.2494934
Detection of S1 spike protein in CD16+ monocytes up to 245 days in SARS-CoV-2-negative post-COVID-19 vaccine syndrome (PCVS) individuals
Abstract
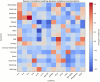
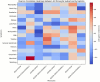
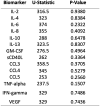
Despite over 13 billion SARS-CoV-2 vaccine doses administered globally, persistent post-vaccination symptoms, termed post-COVID-19 vaccine syndrome (PCVS), resemble post-acute sequelae of COVID-19 (PASC). Symptoms like cardiac, vascular, and neurological issues often emerge shortly after vaccination and persist for months to years, mirroring PASC. We previously showed the S1 subunit of the SARS-CoV-2 spike protein persists in CD16+ monocytes after infection, potentially driving PASC. Approved vaccines (Pfizer, Moderna, Janssen, AstraZeneca) deliver synthetic S1 to elicit immunity, suggesting a shared mechanism. We hypothesized that vaccine-derived S1 persistence in CD16+ monocytes sustains inflammation akin to PASC, contributing to PCVS. We studied 50 individuals with PCVS symptoms lasting over 30 days post-vaccination and 26 asymptomatic controls, using (1) machine learning-based immune profiling to compare cytokine signatures with PASC, (2) flow cytometry to detect S1 in CD16+ monocytes, and (3) LC-MS to confirm S1 across vaccine types. We correlated S1 persistence with symptom duration and inflammation. Prior infection was excluded via clinical history, anti-nucleocapsid antibody tests, and T-detect assays, though definitive tests are lacking. Preliminary findings suggest S1 persistence in CD16+ monocytes and an associated inflammatory profile may contribute to PCVS. Further studies are needed to confirm causality and prevalence.
Keywords: CCR5; COVID-19; PASC; SARS CoV-2 S1 protein; fractalkine; non-classical monocytes.
Plain language summary
SARS CoV-2 S1 Protein in CD16+ Monocytes in Post-COVID-19 Vaccine Syndrome (PCVS).
Conflict of interest statement
Bruce Patterson, Edgar B. Francisco, Emily Long, Christopher Beaty and Amruta Pise are employees of IncellDX. Bruce Patterson is CEO of IncellDX.
Bruce Patterson, Ram Yogendra, John Bream, Eric Osgood, Devon Jeffers and Mark Kreimer are independent contractors of the Chronic COVID Treatment Center (CCTC). CCTC is a private physician practice group that is independent of and receives no funding or support from IncellDX or any other entity.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
