Platelet-Derived Soluble CD40L and Its Impact on Immune Modulation and Anti-IL6R Antibody Treatment Outcome in Rheumatoid Arthritis
- PMID: 40358149
- PMCID: PMC12071919
- DOI: 10.3390/cells14090625
Platelet-Derived Soluble CD40L and Its Impact on Immune Modulation and Anti-IL6R Antibody Treatment Outcome in Rheumatoid Arthritis
Abstract
Background: Platelets (PLTs) from healthy donors (HD) modulate T lymphocyte responses but PLTs from rheumatoid arthritis (RA) patients contribute to persistent systemic inflammation. This suggests that PLTs from RA patients and HD have different immunomodulatory effects.
Methods: Using cell culture, flow cytometry, proteomics, and ELISA, we compared PLTs from HD and RA patients and their effects on T lymphocyte activation and cytokine production.
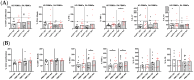
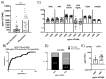
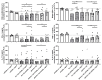
Results: HD PLTs suppressed T lymphocyte proliferation and IFNγ and TNF production, while RA PLTs exhibited reduced suppressive capacity. In the presence of RA PLTs, IFNγ levels correlated with T lymphocyte proliferation, greater disease activity, and anti-citrullinated protein antibodies (ACPA). Proteomic analysis revealed that RA PLTs show upregulation of proteins linked to acute-phase response and complement activation. RA PLTs secreted higher levels of soluble CD40L (sCD40L) and PDGF-BB that correlated with enhanced IFNγ production. Seropositive RA patients had higher levels of sCD40L, and these levels were predictive of disease remission in RA patients treated with anti-IL6R. sCD40L was found to enhance T lymphocyte activation and to contribute to increased pro-inflammatory cytokine production.
Conclusions: This study highlights the diminished ability of RA PLTs to suppress T lymphocyte activation and that sCD40L can be a potential biomarker and therapeutic target in RA.
Keywords: CD40L; T lymphocyte activation; cytokine production; platelet immunomodulation; rheumatoid arthritis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

