The Efficacy of Targeted Monoclonal IgA Antibodies Against Pancreatic Ductal Adenocarcinoma
- PMID: 40358156
- PMCID: PMC12071589
- DOI: 10.3390/cells14090632
The Efficacy of Targeted Monoclonal IgA Antibodies Against Pancreatic Ductal Adenocarcinoma
Abstract
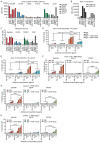
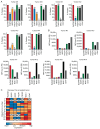
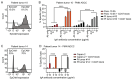
The efficacy of immunotherapy in pancreatic ductal adenocarcinoma (PDAC) remains limited. The tumor microenvironment (TME), characterized by the accumulation of suppressive myeloid cells including neutrophils, attributes to immunotherapy resistance in PDAC. IgA monoclonal antibodies (mAbs) can activate neutrophils to kill tumor cells; this can be further enhanced by blocking the myeloid immune checkpoint CD47. In this study, we investigated the potential of this therapeutic strategy for PDAC. We determined the expression of tumor-associated antigens (TAAs) on PDAC cell lines and fresh patient samples, and the results showed that the TAAs epithelial cell adhesion molecule (EpCAM), trophoblast cell surface antigen 2 (TROP2) and mucin-1 (MUC1), as well as CD47 were consistently expressed on PDAC. In line with this, we showed that IgA mAbs against EpCAM can activate neutrophils to lyse various PDAC cell lines and tumor cells, which can be augmented by addition of CD47 blockade. In addition, we observed that neutrophils were present in patient tumors and expressed the receptor for IgA. In conclusion, our results indicate that a combination of IgA mAb with CD47 blockade is a promising preclinical treatment strategy for PDAC, which merits further investigation.
Keywords: IgA; PDAC; PMNs; mAbs; monoclonal antibodies; neutrophils; pancreatic cancer; pancreatic ductal adenocarcinoma; polymorphonuclear cells.
Conflict of interest statement
L.R., E.M.P., M.E.V., M.E., C.C., K.C.K., G.M.R., I.Q.M., H.C.v.S., K.S., M.P.W.I., L.A.D. and P.A.O. have nothing to disclose. J.H.W.L. is the scientific founder and a shareholder of TigaTx and was supported by Oncode Accelerator, a Dutch National Growth Fund project under grant number NGFOP2201.
Figures


References
-
- Latenstein A.E.J., van der Geest L.G.M., Bonsing B.A., Groot Koerkamp B., Haj Mohammad N., de Hingh I., de Meijer V.E., Molenaar I.Q., van Santvoort H.C., van Tienhoven G., et al. Nationwide trends in incidence, treatment and survival of pancreatic ductal adenocarcinoma. Eur. J. Cancer. 2020;125:83–93. doi: 10.1016/j.ejca.2019.11.002. - DOI - PubMed
-
- Orth M., Metzger P., Gerum S., Mayerle J., Schneider G., Belka C., Schnurr M., Lauber K. Pancreatic ductal adenocarcinoma: Biological hallmarks, current status, and future perspectives of combined modality treatment approaches. Radiat. Oncol. 2019;14:141. doi: 10.1186/s13014-019-1345-6. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

