Prolactin Mediates Distinct Time Course Regulation of Tyrosine Hydroxylase Phosphorylation and Gene Expression in Tuberoinfundibular Dopaminergic Neurons of Female Rats
- PMID: 40358166
- PMCID: PMC12071785
- DOI: 10.3390/cells14090642
Prolactin Mediates Distinct Time Course Regulation of Tyrosine Hydroxylase Phosphorylation and Gene Expression in Tuberoinfundibular Dopaminergic Neurons of Female Rats
Abstract
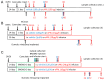
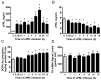
Prolactin (PRL) regulates its own secretion by short-loop feedback to tuberoinfundibular dopaminergic (TIDA) neurons. PRL-induced cellular mechanisms in the regulation of tyrosine hydroxylase (TH) are not completely understood. The objectives were to (1) examine PRL-induced, time-dependent hypothalamic changes in JAK2-STAT5B signaling, TH activity, TH phosphorylation state and Th mRNA levels, and (2) evaluate direct influences of PRLR-STAT5B signaling on Th promoter activity. Ovariectomized rats were administered ovine PRL. JAK2 and STAT5 phosphorylation in the mediobasal hypothalamus peaked at 15 and 30-60 min, respectively. TH Ser40 phosphorylation in the median eminence was increased between 2 and 72 h, correlating with increased dihydroxyphenylalanine (DOPA) accumulation. Th mRNA levels in TIDA neurons were unchanged up to 72 h but elevated by 7 days. PRL did not alter Th promoter activity in CAD cells, and STAT5B did not bind three putative Gamma Interferon Activation Sites (GAS) elements. We conclude that PRL initiates an integrated cascade of cellular mechanisms in TIDA neurons, including JAK2-STAT5B activation, TH Ser40 phosphorylation coupled to increased TH activity, followed by a delayed rise in Th gene expression. PRL-induced changes in Th gene expression are not the result of STAT5-mediated transactivation but likely result from enduring changes in TIDA neuronal activity.
Keywords: JAK2; STAT5; TIDA; dopamine; gene expression; phosphorylation; prolactin; prolactin receptor signaling; promoter activity; tyrosine hydroxylase.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

