Nardostachys jatamansi Extract and Nardosinone Exert Neuroprotective Effects by Suppressing Glucose Metabolic Reprogramming and Modulating T Cell Infiltration
- PMID: 40358168
- PMCID: PMC12071694
- DOI: 10.3390/cells14090644
Nardostachys jatamansi Extract and Nardosinone Exert Neuroprotective Effects by Suppressing Glucose Metabolic Reprogramming and Modulating T Cell Infiltration
Abstract
Background: Nardostachys jatamansi DC. (Gansong), a widely utilized herb in traditional Chinese medicine, has been historically employed in the management of various neuropsychiatric disorders. Nardosinone (Nar), a sesquiterpenoid compound, has been identified as one of the principal bioactive constituents of N. jatamansi. This study investigated the effects of ethyl acetate extract (NJ-1A) from N. jatamansi and its active constituent nardosinone on neuroinflammatory mediator release, glucose metabolic reprogramming, and T cell migration using both in vitro and in vivo experimental models.
Methods: Lipopolysaccharide(LPS)-induced BV-2 microglial cells and a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine/probenecid (MPTP/p)-induced male C57BL/6N mouse chronic model of Parkinson's disease were applied.
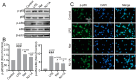
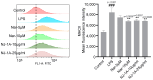
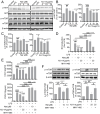
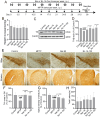
Results: Both NJ-1A and Nar could significantly suppress LPS-induced production of M1 pro-inflammatory factors or markers in microglia and could inhibit the glycolytic process and promote oxidative phosphorylation via the AKT/mTOR signaling pathway. Furthermore, they exhibited the capacity to attenuate chemokine release from activated microglia, consequently reducing T cell migration. In vivo experiments revealed that NJ-1A and Nar effectively inhibited microglial activation, diminished T cell infiltration, and mitigated the loss of tyrosine hydroxylase (TH)-positive dopaminergic neurons in the substantia nigra of MPTP-induced mice.
Conclusions: NJ-1A and nardosinone exert neuroprotective effects through the modulation of microglial polarization states, regulation of metabolic reprogramming, and suppression of T cell infiltration.
Keywords: Nardostachys jatamansi; Parkinson’s disease; T cell infiltration; metabolic reprogramming; microglia; nardosinone; neuroinflammation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

