Isolation and Characterization of Mouse Choroidal Melanocytes and Their Proinflammatory Characteristics
- PMID: 40358170
- PMCID: PMC12071734
- DOI: 10.3390/cells14090646
Isolation and Characterization of Mouse Choroidal Melanocytes and Their Proinflammatory Characteristics
Abstract
Melanocytes are a major cellular component of the choroid which aids in the maintenance of choroidal integrity and vision. Unfortunately, our knowledge regarding the cell autonomous melanocyte function, in preserving choroidal health and the ocular pathologies associated with choroidal dysfunction, remain largely unknown. The ability to culture melanocytes has advanced our knowledge regarding the origin and function of these cells in choroidal homeostasis and vision. However, the culture of murine choroid melanocytes has not been previously reported. Here, we describe a method for the isolation of melanocytes from the mouse choroid, as well as the delineation of many of their cellular characteristics, including the expression of various cell-specific markers, cell adhesion molecules, melanogenic capacity, and inflammatory responses to various extracellular stressors. Unraveling the molecular mechanisms that regulate melanocyte functions will advance our understanding of their role in choroidal homeostasis and how alterations in these functions impact ocular diseases that compromise vision.
Keywords: VEGF receptors; age-related macular degeneration; c-Kit; cell adhesion molecules; choroid; inflammation; integrins; stem cell factor.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


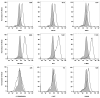

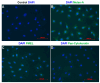
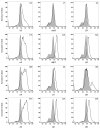
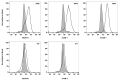




References
-
- Adameyko I., Lallemend F., Furlan A., Zinin N., Aranda S., Kitambi S.S., Blanchart A., Favaro R., Nicolis S., Lübke M., et al. Sox2 and mitf cross-regulatory interactions consolidate progenitor and melanocyte lineages in the cranial neural crest. Development. 2012;139:397–410. doi: 10.1242/dev.065581. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- P30 EY016665/EY/NEI NIH HHS/United States
- EY034646/EY/NEI NIH HHS/United States
- P30 CA014520/CA/NCI NIH HHS/United States
- R01 EY030076/EY/NEI NIH HHS/United States
- AMD Research Award/Arthur and Nancy Nesbit AMD fund
- AMD Research Award/Carl Marshall Reeves & Mildred Almen Reeves Foundation
- EY030076/EY/NEI NIH HHS/United States
- Endoument/RRF/Daniel Albert Chair
- Endoument/Retina Research Foundation
- R21 EY034646/EY/NEI NIH HHS/United States
- Unrestricted Award to the Deaprtment of Ophthalmology and visual Sciences/Research to Prevent Blindness
- AMD Research Award/Pat and Jay Smith AMD Innovation Fund
LinkOut - more resources
Full Text Sources

