The Six-Transmembrane Epithelial Antigen of the Prostate (STEAP) 3 Regulates the Myogenic Differentiation of Yunan Black Pig Muscle Satellite Cells (MuSCs) In Vitro via Iron Homeostasis and the PI3K/AKT Pathway
- PMID: 40358178
- PMCID: PMC12071230
- DOI: 10.3390/cells14090656
The Six-Transmembrane Epithelial Antigen of the Prostate (STEAP) 3 Regulates the Myogenic Differentiation of Yunan Black Pig Muscle Satellite Cells (MuSCs) In Vitro via Iron Homeostasis and the PI3K/AKT Pathway
Abstract
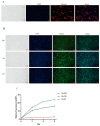
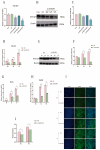
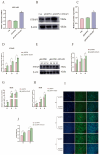
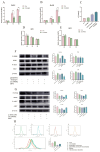
The myogenic differentiation of muscle satellite cells (MuSCs) is an important biological process that plays a key role in the regeneration and repair of skeletal muscles. However, the mechanisms regulating myoblast myogenesis require further investigation. In this study, we found that STEAP3 is involved in myogenic differentiation based on the Yunan black pig MuSCs model in vitro using cell transfection and other methods. Furthermore, the expression of myogenic differentiation marker genes MyoG and MyoD and the number of myotubes formed by the differentiation of cells from the si-STEAP3 treated group were significantly decreased but increased in the STEAP3 overexpression group compared to that in the control group. STEAP3 played a role in iron ion metabolism, affecting myogenic differentiation via the uptake of iron ions and enhancing IRP-IRE homeostasis. STEAP3 also activated the PI3K/AKT pathway, thus promoting myoblast differentiation of Yunan black pig MuSCs. The results of this study showed that STEAP3 overexpression increased intracellular iron ion content and activated the homeostatic IRP-IRE system to regulate intracellular iron ion metabolism.
Keywords: MuSCs myogenic differentiation; PI3K/AKT pathway; STEAP3; iron homeostasis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Effect of IGF1 on Myogenic Proliferation and Differentiation of Bovine Skeletal Muscle Satellite Cells Through PI3K/AKT Signaling Pathway.Genes (Basel). 2024 Nov 21;15(12):1494. doi: 10.3390/genes15121494. Genes (Basel). 2024. PMID: 39766763 Free PMC article.
-
Formononetin ameliorates muscle atrophy by regulating myostatin-mediated PI3K/Akt/FoxO3a pathway and satellite cell function in chronic kidney disease.J Cell Mol Med. 2021 Feb;25(3):1493-1506. doi: 10.1111/jcmm.16238. Epub 2021 Jan 6. J Cell Mol Med. 2021. PMID: 33405354 Free PMC article.
-
WDR13 promotes the differentiation of bovine skeletal muscle-derived satellite cells by affecting PI3K/AKT signaling.Cell Biol Int. 2019 Jul;43(7):799-808. doi: 10.1002/cbin.11160. Epub 2019 May 27. Cell Biol Int. 2019. PMID: 31050064
-
Postnatal skeletal muscle myogenesis governed by signal transduction networks: MAPKs and PI3K-Akt control multiple steps.Biochem Biophys Res Commun. 2023 Nov 19;682:223-243. doi: 10.1016/j.bbrc.2023.09.048. Epub 2023 Sep 27. Biochem Biophys Res Commun. 2023. PMID: 37826946 Review.
-
Muscle stem cells as immunomodulator during regeneration.Curr Top Dev Biol. 2024;158:221-238. doi: 10.1016/bs.ctdb.2024.01.010. Epub 2024 Feb 15. Curr Top Dev Biol. 2024. PMID: 38670707 Free PMC article. Review.
References
-
- Olenic M., Deelkens C., Heyman E., De Vlieghere E., Zheng X., van Hengel J., De Schauwer C., Devriendt B., De Smet S., Thorrez L. Review: Livestock cell types with myogenic differentiation potential: Considerations for the development of cultured meat. Animal. 2024;19:101242. doi: 10.1016/j.animal.2024.101242. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

