Targeting Glioma Stem Cells: Therapeutic Opportunities and Challenges
- PMID: 40358199
- PMCID: PMC12072158
- DOI: 10.3390/cells14090675
Targeting Glioma Stem Cells: Therapeutic Opportunities and Challenges
Abstract
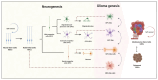
Glioblastoma (GBM), or grade 4 glioma, is the most common and aggressive primary brain tumor in adults with a median survival of 15 months. Increasing evidence suggests that GBM's aggressiveness, invasiveness, and therapy resistance are driven by glioma stem cells (GSCs), a subpopulation of tumor cells that share molecular and functional characteristics with neural stem cells (NSCs). GSCs are heterogeneous and highly plastic. They evade conventional treatments by shifting their state and entering in quiescence, where they become metabolically inactive and resistant to radiotherapy and chemotherapy. GSCs can exit quiescence and be reactivated to divide into highly proliferative tumor cells which contributes to recurrence. Understanding the molecular mechanisms regulating the biology of GSCs, their plasticity, and the switch between quiescence and mitotic activity is essential to shape new therapeutic strategies. This review examines the latest evidence on GSC biology, their role in glioblastoma progression and recurrence, emerging therapeutic approaches aimed at disrupting their proliferation and survival, and the mechanisms underlying their resistance to therapy.
Keywords: glioma stem cells; plasticity; quiescence; therapeutic targets; therapy resistance.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Hunting glioblastoma recurrence: glioma stem cells as retrospective targets.Am J Physiol Cell Physiol. 2025 Mar 1;328(3):C1045-C1061. doi: 10.1152/ajpcell.00344.2024. Epub 2025 Jan 16. Am J Physiol Cell Physiol. 2025. PMID: 39818986 Review.
-
MELK-dependent FOXM1 phosphorylation is essential for proliferation of glioma stem cells.Stem Cells. 2013 Jun;31(6):1051-63. doi: 10.1002/stem.1358. Stem Cells. 2013. PMID: 23404835 Free PMC article.
-
A novel drug conjugate, NEO212, targeting proneural and mesenchymal subtypes of patient-derived glioma cancer stem cells.Cancer Lett. 2016 Feb 28;371(2):240-50. doi: 10.1016/j.canlet.2015.11.040. Epub 2015 Dec 9. Cancer Lett. 2016. PMID: 26683773
-
Targeting role of glioma stem cells for glioblastoma multiforme.Curr Med Chem. 2013;20(15):1974-84. doi: 10.2174/0929867311320150004. Curr Med Chem. 2013. PMID: 23317162 Review.
-
Dedifferentiation of Glioma Cells to Glioma Stem-like Cells By Therapeutic Stress-induced HIF Signaling in the Recurrent GBM Model.Mol Cancer Ther. 2016 Dec;15(12):3064-3076. doi: 10.1158/1535-7163.MCT-15-0675. Epub 2016 Oct 7. Mol Cancer Ther. 2016. PMID: 27765847 Free PMC article.
References
-
- Fernandes C., Costa A., Osório L., Lago R.C., Linhares P., Carvalho B., Caeiro C. Current Standards of Care in Glioblastoma Therapy. Exon Publications; Brisbane, Australia: 2017. pp. 197–241. - PubMed
-
- Murat A., Migliavacca E., Gorlia T., Lambiv W.L., Shay T., Hamou M.-F., De Tribolet N., Regli L., Wick W., Kouwenhoven M.C. Stem cell–related “self-renewal” signature and high epidermal growth factor receptor expression associated with resistance to concomitant chemoradiotherapy in glioblastoma. J. Clin. Oncol. 2008;26:3015–3024. doi: 10.1200/JCO.2007.15.7164. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

