Cambridge Neoadjuvant Cancer of the Prostate (CANCAP03): A Window Study into the Effects of Olaparib ± Degarelix in Primary Prostate Cancer
- PMID: 40358364
- PMCID: PMC7617790
- DOI: 10.1158/1078-0432.CCR-24-1304
Cambridge Neoadjuvant Cancer of the Prostate (CANCAP03): A Window Study into the Effects of Olaparib ± Degarelix in Primary Prostate Cancer
Abstract
Purpose: The purpose was to investigate combined PARP and androgen inhibition in primary prostate cancer and understand the biological mechanisms underlying clinical efficacy, especially in the absence of mutations in homologous recombination (HR) repair pathways.
Patients and methods: The primary objective was to measure PARP inhibition, and the secondary objectives were to assess safety and feasibility. Participants received olaparib for 2 weeks before prostatectomy and were randomly assigned or not assigned (1:1) to degarelix. We analyzed diagnostic biopsy and radical prostatectomy samples for PARylated protein expression using IHC. Exploratory analyses included tumor gene sequencing, mutation analysis, and RNA sequencing (RNA-seq) using both bulk and single-cell RNA-seq performed on pretreatment and posttreatment tissues.
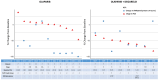
Results: PARylated protein expression was significantly reduced in both cohorts, with no drug-related delays in radical prostatectomy. The gene set enrichment analysis identified distinct treatment response signatures related to olaparib in both cohorts and showed downregulation of androgen response genes after olaparib + degarelix treatment.Transcript profiling revealed an upregulation of the p53 hallmark, which was more pronounced with the combination treatment. Canonical cell-cycle progression hallmarks, including E2F targets and the G2-M checkpoint, were suppressed across all cases, correlating with a HR-deficient transcriptional signature. Single-nuclear RNA-seq indicated a greater increase in inflammatory response pathway activity within tumor epithelia after combination treatment.
Conclusions: Transcriptomic analysis identified common hallmark alterations reflecting the combined impact of PARP inhibitor and androgen blockade on cell-cycle progression. We observed a shared phenotypic response to combination therapy across prostate cancers without known HR repair gene alterations. This suggests alternative mechanisms rather than antiandrogen-induced HR deficiency.
©2025 American Association for Cancer Research.
Conflict of interest statement
SP has received research funding from Astra Zeneca and acted as a consultant to Astra Zeneca
HD has received research funding from Astra Zeneca
EH, NC, SG, MS, AR, LM, RH are employees of Astra Zeneca and hold stock options
All remaining authors have declared no conflicts of interest
Figures



References
-
- Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–263. - PubMed
-
- Gillessen S, Bossi A, Davis ID, et al. Management of Patients with Advanced Prostate Cancer. Part I: Intermediate-/High-risk and Locally Advanced Disease, Biochemical Relapse, and Side Effects of Hormonal Treatment: Report of the Advanced Prostate Cancer Consensus Conference 2022. Eur Urol. 2023;83(3):267–293. doi: 10.1016/j.eururo.2022.11.002. - DOI - PMC - PubMed
-
- Gillessen S, Bossi A, Davis ID, et al. Management of patients with advanced prostate cancer-metastatic and/or castration-resistant prostate cancer: Report of the Advanced Prostate Cancer Consensus Conference (APCCC) 2022. Eur J Cancer. 2023;185:178–215. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

