The Fecal Mycobiome in Chronic Pancreatitis Is Characterized by an Increase in Candida species and Nakaseomyces
- PMID: 40358410
- PMCID: PMC12330351
- DOI: 10.14309/ctg.0000000000000855
The Fecal Mycobiome in Chronic Pancreatitis Is Characterized by an Increase in Candida species and Nakaseomyces
Abstract
Introduction: The exocrine pancreas is an important determinant of the intestinal microbiome composition and stability. Although chronic pancreatitis (CP) is known to severely affect the bacterial community, its impact on the intestinal mycobiome is currently unknown.
Methods: A total of 93 patients with clinical and imaging evidence of CP were prospectively recruited and compared with 2 equally sized matched control cohorts. One control group was matched for age, sex, body mass index, and smoking (Con-1), and the other additionally for exocrine pancreatic function (stool elastase) and diabetes (Con-2). Fecal samples were collected from all 279 individuals to determine the fecal mycobiome by internal transcribed spacer 2 sequencing.
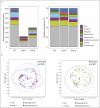
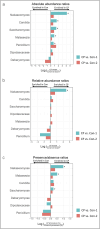
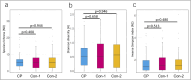
Results: In CP patients, fungal reads were increased (3.7-fold and 2.0-fold) as compared with Con-1 and Con-2. In comparison with Con-1, CP patients demonstrated higher total abundance of Candida (4.5-fold, q = 0.009) and higher mean relative abundance (11.4% vs 1.0%, q = 0.014) and presence (25.8% vs 9.7%, q = 0.025) of Nakaseomyces . In contrast to Con-2, CP patients showed higher Candida total abundance (1.9-fold, P = 0.016) which was, however, not significant after correction for multiple testing ( q = 0.056).
Discussion: Not only the microbiome but also the mycobiome in CP patients is characterized by distinct changes, with higher abundances of Candida or Nakaseomyces . Exocrine pancreatic dysfunction in CP patients likely contributes to this observation. This may result in increased rates of fungal infections, chronic inflammation, and could be contributing to the development of pancreatic cancer.
Keywords: acute pancreatitis; fungeome; microbiome; microbiota; pancreatic cancer.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of The American College of Gastroenterology.
Conflict of interest statement
Figures

References
-
- Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol Rev 2001;81(3):1031–64. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

