Evaluating Cost-Effective Strategies for Asymptomatic Microhematuria Diagnosis: A Risk-Based Alternative to the American Urological Association Guidelines
- PMID: 40358418
- PMCID: PMC12232114
- DOI: 10.1002/jso.28148
Evaluating Cost-Effective Strategies for Asymptomatic Microhematuria Diagnosis: A Risk-Based Alternative to the American Urological Association Guidelines
Abstract
Background and objectives: The American Urological Association (AUA) guidelines recommend evaluating asymptomatic microhematuria (MH) at ≥ 3 red blood cells per high powered field (RBCs/hpf), resulting in significant costs with limited bladder cancer detections. This study evaluates alternative diagnostic strategies to improve the cost-effectiveness of asymptomatic MH evaluation.
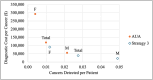
Methods: The cost-effectiveness analysis compared three alternative strategies: Strategy 1 (cystoscopy at ≥ 26 RBCs/hpf) was compared to a 3 RBCs/hpf threshold, while Strategy 2 (cystoscopy and renal ultrasound at ≥ 3 RBCs/hpf) and Strategy 3 (cystoscopy and renal ultrasound at ≥ 26 RBCs/hpf) were compared to the AUA guidelines. Total costs, cost per patient evaluated, costs per cancer detected, and incremental cost-effectiveness ratios (ICERs) were calculated.
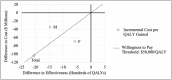
Results: Strategy 3 minimized costs without significantly reducing early cancer detection rates. It was cost-effective for females (ICER = $120,649) and the total sample (ICER = $50,648) but not specifically for males (ICER = $23,326). Strategies 1 and 2 yielded lower cost savings and were less efficient.
Conclusions: Strategy 3-performing cystoscopy and renal ultrasound for higher-risk patients ( ≥ 26 RBCs/hpf)-offers a more cost-effective approach than the AUA guidelines, particularly for women. Future studies should incorporate additional patient variables and diagnostic test characteristics.
Keywords: bladder cancer; cost‐effectiveness; cystoscopy; microhematuria.
© 2025 The Author(s). Journal of Surgical Oncology published by Wiley Periodicals LLC.
Figures





References
-
- Williams S. B., Howard L. E., Foster M. L., et al., “Estimated Costs and Long‐Term Outcomes of Patients With High‐Risk Non–Muscle‐Invasive Bladder Cancer Treated With Bacillus Calmette‐Guérin in the Veterans Affairs Health System,” JAMA Network Open 4, no. 3 (2021): e213800, 10.1001/jamanetworkopen.2021.3800. - DOI - PMC - PubMed
-
- Cambier S., Sylvester R. J., Collette L., et al., “EORTC Nomograms and Risk Groups for Predicting Recurrence, Progression, and Disease‐Specific and Overall Survival in Non–Muscle‐Invasive Stage Ta–T1 Urothelial Bladder Cancer Patients Treated With 1–3 Years of Maintenance Bacillus Calmette‐Guérin,” European Urology 69, no. 1 (2016): 60–69, 10.1016/j.eururo.2015.06.045. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

