Musclin Counteracts Skeletal Muscle Dysfunction and Exercise Intolerance in Heart Failure With Preserved Ejection Fraction
- PMID: 40358602
- PMCID: PMC12165570
- DOI: 10.1161/CIRCHEARTFAILURE.124.012350
Musclin Counteracts Skeletal Muscle Dysfunction and Exercise Intolerance in Heart Failure With Preserved Ejection Fraction
Abstract
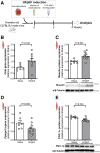
Background: Exercise intolerance is a hallmark of heart failure with preserved ejection fraction (HFpEF) and is characterized by skeletal muscle (SkM) dysfunction with impaired oxidative capacity. To maintain oxidative capacity, the SkM secretes myokines such as musclin, which has been shown to potentiate NP (natriuretic peptide) signaling and induce PGC-1α (peroxisome proliferator-activated receptor-γ coactivator-1 alpha) signaling. We sought to investigate the role of musclin in SkM dysfunction in HFpEF. For this study, we selected the oxidative-predominant SkM soleus in HFpEF mice and vastus lateralis from patients with HFpEF.
Methods: Using the SAUNA model, mice underwent HFpEF induction by uninephrectomy, d-aldosterone infusion, and 1% sodium chloride drinking water for 4 weeks. Exogenous musclin was given to HFpEF mice every 2 days during the last 2 weeks of HFpEF induction. Molecular analyses were conducted on blood samples and SkM from HFpEF mice and patients with HFpEF.
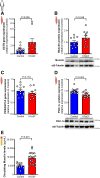
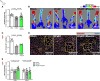
Results: In HFpEF mice and patients with HFpEF, increased musclin expression was accompanied by decreased cyclic guanosine monophosphate levels and PGC-1α expression in SkM, suggesting impaired NP signaling. Exogenous administration of musclin in mice with HFpEF demonstrated augmented circulating musclin levels and potentiated NP signaling in SkM as shown by increased PKG1 (protein kinase G1) activity and PGC-1α expression. This was associated with a transition from type-2A to type-1 fiber (type-1 has more endurance) and increased succinate dehydrogenase activity, hindlimb blood flow, and capillary density in the soleus muscle. Exogenous musclin also mitigated cardiac hypertrophy without affecting blood pressure or diastolic function. Most importantly, HFpEF mice treated with musclin demonstrated improved functional and exercise capacity.
Conclusions: Musclin mediates beneficial effects in SkM and heart with improved exercise capacity likely by improving oxidative capacity in SkM. Future studies are warranted to address the therapeutic efficacy of exogenous musclin in humans with HFpEF.
Keywords: exercise tolerance; heart failure; muscle, skeletal; myokines; natriuretic peptides.
Conflict of interest statement
Dr Zamani consulted for Pfizer and Vyaire. Dr Chirinos is an editorial board member of the American Heart Association, the American College of Cardiology, Elsevier, and Wiley. He consulted for Bayer, Fukuda Denshi, Bristol Myers Squibb, JNJ, Edwards Life Sciences, Merck, and NGM Biopharmaceuticals. Dr Sam is a full-time employee of Eli Lilly and Co, Indianapolis, IN. None of the work was funded nor supported by Eli Lilly and Co. The other authors report no conflicts.
Figures





Comment in
-
Musclin and HFpEF: Unlocking Skeletal Muscle Potential to Improve Exercise Tolerance.Circ Heart Fail. 2025 Jun;18(6):e013130. doi: 10.1161/CIRCHEARTFAILURE.125.013130. Epub 2025 May 6. Circ Heart Fail. 2025. PMID: 40326355 No abstract available.
References
-
- Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, et al. ; DELIVER Trial Committees and Investigators. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387:1089–1098. doi: 10.1056/NEJMoa2206286 - PubMed
-
- Kosiborod MN, Abildstrøm SZ, Borlaug BA, Butler J, Rasmussen S, Davies M, Hovingh GK, Kitzman DW, Lindegaard ML, Møller DV, et al. ; STEP-HFpEF Trial Committees and Investigators. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N Engl J Med. 2023;389:1069–1084. doi: 10.1056/NEJMoa2306963 - PubMed
-
- Pandey A, Shah SJ, Butler J, Kellogg DL, Jr, Lewis GD, Forman DE, Mentz RJ, Borlaug BA, Simon MA, Chirinos JA, et al. Exercise intolerance in older adults with heart failure with preserved ejection fraction: JACC state-of-the-art review. J Am Coll Cardiol. 2021;78:1166–1187. doi: 10.1016/j.jacc.2021.07.014 - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

