Characterization of the aryl hydrocarbon receptor as a potential candidate to improve cancer T cell therapies
- PMID: 40358739
- PMCID: PMC12075070
- DOI: 10.1007/s00262-025-04065-5
Characterization of the aryl hydrocarbon receptor as a potential candidate to improve cancer T cell therapies
Abstract
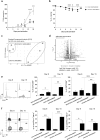
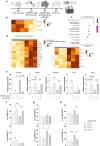
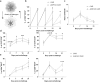
The efficacy of T-cell-based cancer therapies can be limited by the tumor microenvironment which can lead to T cell dysfunction. Multiple studies, particularly in murine models, have demonstrated the capacity of the aryl hydrocarbon receptor (AHR) to negatively regulate antitumor T cell functions. AHR is a cytoplasmic receptor and transcription factor that was originally identified as a xenobiotic sensor, but has since been shown to play a significant role in the gene regulation of various immune cells, including T cells. Given the insights from murine studies, AHR emerges as a promising candidate to invalidate for optimizing T cell-based cancer therapies. However, the controversial role of AHR in human T cells underscores the need for a more comprehensive characterization of AHR expressing T cells. This study aims to investigate the regulatory mechanisms of AHR in human T cell biology to better understand its impact on reducing antitumor immune responses. Here, we knocked-out AHR in human T cells using CRISPR-Cas9 technology to characterize AHR's function in an in vitro chronic stimulation model. Engineered T cells exhibited enhanced effector- and memory-like profiles and expressed reduced amount of CD39 and TIGIT. AHR knockout enhanced human CAR-T cells' functionality and persistence upon tumor chronic stimulation. Collectively, these results highlight the role of AHR in human CAR-T cells efficiency.
Keywords: AHR; CAR-T cell therapy; CRISPR-Cas9; T cell dysfunction.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare no competing interests. Ethical approval: The studies involving humans were approved by CODECOH (collection agreement number AC-2020-4129). The studies were conducted in accordance with the local legislation and institutional requirements. Consent to participate: The participants provided their written informed consent to participate in this study.
Figures




References
-
- De Castro V, Galaine J, Loyon R, Godet Y (2024) Godet et al CRISPR-Cas gene knockouts to optimize engineered T cells for cancer immunotherapy. Cancer Gene Ther. 10.1038/s41417-024-00771-x - PubMed
MeSH terms
Substances
Grants and funding
- 2022-0047/Ligue Contre le Cancer
- 2022-0047/Ligue Contre le Cancer
- 2022-0047/Ligue Contre le Cancer
- 2022-0047/Ligue Contre le Cancer
- 2022-0047/Ligue Contre le Cancer
- 2022-0047/Ligue Contre le Cancer
- 2022-0047/Ligue Contre le Cancer
- 2022-0047/Ligue Contre le Cancer
- 2022-0047/Ligue Contre le Cancer
- 2022-0047/Ligue Contre le Cancer
- BFC000802/BioImp project
- BFC000802/BioImp project
- BFC000802/BioImp project
- BFC000802/BioImp project
- BFC000802/BioImp project
- BFC000802/BioImp project
- BFC000802/BioImp project
- BFC000802/BioImp project
- BFC000802/BioImp project
- BFC000802/BioImp project
LinkOut - more resources
Full Text Sources
Medical
Research Materials

